

|
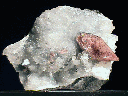
The Zeolites are a popular group of minerals for collectors and an important group of minerals for industrial and other purposes. They combine rarity, beauty, complexity and unique crystal habits. Typically forming in the cavities (or vesicles) of volcanic rocks, zeolites are the result of very low grade metamorphism. Some form from just subtle amounts of heat and pressure and can just barely be called metamorphic while others are found in obviously metamorphic regimes. Zeolite crystals have been grown on board the space shuttle and are undergoing extensive research into their formation and unique properties.
The zeolites are framework silicates consisting of interlocking tetrahedrons of SiO4 and AlO4.
In order to be a zeolite the ratio (Si +Al)/O must equal 1/2.
The alumino-silicate structure is negatively charged and attracts the positive cations that reside within.
Unlike most other
tectosilicates, zeolites have large vacant spaces or
cages in their structures that allow space for large cations such as sodium, potassium, barium and calcium and even relatively large molecules and cation groups such as water, ammonia, carbonate ions and nitrate ions.
In the more useful zeolites, the spaces are interconnected and form long wide channels of varying sizes depending on the mineral.
These channels allow the easy movement of the resident ions and molecules into and out of the structure.
Zeolites are characterized by their ability to lose and absorb water without damage to their crystal structures.
The large channels explain the consistent low
Zeolites have many useful purposes. They can perform ion exchange, filtering, odor removal, chemical sieve and gas absorption tasks. The most well known use for zeolites is in water softeners. Calcium in water can cause it to be "hard" and capable of forming scale and other problems. Zeolites charged with the much less damaging sodium ions can allow the hard water to pass through its structure and exchange the calcium for the sodium ions. This process is reversable. In a similar way zeolites can absorb ions and molecules and thus act as a filter for odor control, toxin removal and as a chemical sieve.
Zeolites can have the water in their structures driven off by heat with the basic structure left intact. Then other solutions can be pushed through the structure. The zeolites can then act as a delivery system for the new fluid. This process has applications in medicine, livestock feeds and other types of research. Zeolites added to livestock feed have been shown to absorb toxins that are damaging and even fatal to the growth of the animals, while the basic structure of the zeolite is biologically neutral. Aquarium hobbyists are seeing more zeolite products in pet stores as zeolites make excellent removers of ammonia and other toxins. Most municipal water supplies are processed through zeolites before public consumption. These uses of zeolites are extremely important for industry, although synthetic zeolites are now doing the bulk of the work.
Zeolites have basically three different structural variations.
- There are chain-like structures whose minerals form acicular or needle-like prismatic crystals, ie natrolite.
- Sheet-like structures where the crystals are flattened platy or tabular with usually good basal cleavages, ie heulandite.
- And framework structures where the crystals are more equant in dimensions, ie Chabazite.
These variations make the zeolite group very diverse, crystal habit-wise. Otherwise zeolites are typically soft to moderately hard, light in density, transparent to translucent and have similar origins. There are about 45 natural minerals that are recognized members of the Zeolite Group. Industrially speaking, the term zeolite includes natural silicate zeolites, synthetic materials and phosphate minerals that have a zeolite like structure. The complexity of this combined group is extensive with over 120 structural variations and more are being discovered or made every year. Collecting zeolites can be very enjoyable and fulfilling.
These are the members of the Zeolite Group:
- The Analcime Family:
- Analcime (Hydrated Sodium Aluminum Silicate)
Pollucite (Hydrated Cesium Sodium Aluminum Silicate)Wairakite (Hydrated Calcium Sodium Aluminum Silicate)
Bellbergite (Hydrated Potassium Barium Strontium Sodium Aluminum Silicate)Bikitaite (Hydrated Lithium Aluminum Silicate)Boggsite (Hydrated calcium Sodium Aluminum Silicate)Brewsterite (Hydrated Strontium Barium Sodium Calcium Aluminum Silicate)- The Chabazite Family:
- Chabazite (Hydrated Calcium Aluminum Silicate)
Willhendersonite (Hydrated Potassium Calcium Aluminum Silicate)
Cowlesite (Hydrated Calcium Aluminum Silicate)Dachiardite (Hydrated calcium Sodium Potassium Aluminum Silicate)- Edingtonite (Hydrated Barium Calcium Aluminum Silicate)
- Epistilbite (Hydrated Calcium Aluminum Silicate)
- Erionite (Hydrated Sodium Potassium Calcium Aluminum Silicate)
Faujasite (Hydrated Sodium Calcium Magnesium Aluminum Silicate)Ferrierite (Hydrated Sodium Potassium Magnesium Calcium Aluminum Silicate)- The Gismondine Family:
Amicite (Hydrated Potassium Sodium Aluminum Silicate)Garronite (Hydrated Calcium Aluminum Silicate)Gismondine (Hydrated Barium Calcium Aluminum Silicate)Gobbinsite (Hydrated Sodium Potassium Calcium Aluminum Silicate)
- Gmelinite (Hydrated Sodium Calcium Aluminum Silicate)
Gonnardite (Hydrated Sodium Calcium Aluminum Silicate)- Goosecreekite (Hydrated Calcium Aluminum Silicate)
- The Harmotome Family:
- Harmotome (Hydrated Barium Potassium Aluminum Silicate)
- Phillipsite (Hydrated Potassium Sodium Calcium Aluminum Silicate)
Wellsite (Hydrated Barium Calcium Potassium Aluminum Silicate)
- The Heulandite Family:
- Clinoptilolite (Hydrated Sodium Potassium Calcium Aluminum Silicate)
- Heulandite (Hydrated Sodium Calcium Aluminum Silicate)
- Laumontite (Hydrated Calcium Aluminum Silicate)
Levyne (Hydrated Calcium Sodium Potassium Aluminum Silicate)Mazzite (Hydrated Potassium Sodium Magnesium Calcium Aluminum Silicate)Merlinoite (Hydrated Potassium Sodium Calcium Barium Aluminum Silicate)Montesommaite (Hydrated Potassium Sodium Aluminum Silicate)- Mordenite (Hydrated Sodium Potassium Calcium Aluminum Silicate)
- The Natrolite Family:
Offretite (Hydrated Calcium Potassium Magnesium Aluminum Silicate)Paranatrolite (Hydrated Sodium Aluminum Silicate)Paulingite (Hydrated Potassium Calcium Sodium Barium Aluminum Silicate)Perlialite (Hydrated Potassium Sodium Calcium Strontium Aluminum Silicate)- The Stilbite Family:
Barrerite (Hydrated Sodium Potassium Calcium Aluminum Silicate)- Stilbite (Hydrated Sodium Calcium Aluminum Silicate)
- Stellerite (Hydrated Calcium Aluminum Silicate)
- Thomsonite (Hydrated Sodium Calcium Aluminum Silicate)
Tschernichite (Hydrated Calcium Aluminum Silicate)Yugawaralite (Hydrated Calcium Aluminum Silicate)











