THE MINERAL NATROLITE
THE MINERAL NATROLITE
- Chemistry: Na2Al2Si3O10-2H2O, Hydrated sodium aluminum silicate
- Class: Silicates
- Subclass: Tektosilicates
- Group: Zeolites
- Uses: mineral specimen and chemical filter
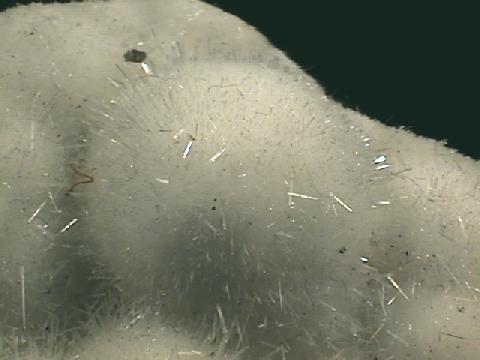
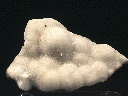
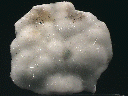
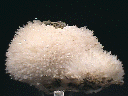
Specimens
Natrolite's structure has a typical zeolite openness about it that allows large ions and molecules to reside and actually move around inside the overall framework. The structure contains open channels that allow water and large ions to travel into and out of the crystal structure. The size of these channels controls the size of the molecules or ions, and therefore zeolites like natrolite can act as a chemical sieve. Natrolite's structure contains chains of silicate tetrahedrons aligned in one direction; this produces the needle-like crystals. Its cleavage results from the weaker bonds between the chains.
Natrolite, a sodium zeolite, scolecite, a calcium zeolite, and mesolite, a calcium and sodium zeolite, are closely related and sometimes found together. The presence of calcium in two of the minerals makes the structure slightly different from that of natrolite; it is altered from an orthorhombic symmetry to a monoclinic symmetry. However, the twinning of scolecite and mesolite often make them appear orthorhombic. All three minerals are referred to as "chain" or "needle" zeolites. They are similar and difficult to distinguish when in clusters with radiating, acicular habits. Natrolite tends to forms thin crystals with pyramidal terminations, but mesolite's fiber-like crystals are usually the thinnest of the three. Scolecite's larger crystals tend to be more robust and durable. These characteristics are only generalities, though, and can not be used as dependable identifying traits; absolute identification cannot be made by ordinary means.
PHYSICAL CHARACTERISTICS:
- Color is clear or white; also tinted yellow and brown.
- Luster is vitreous to dull on some compact masses.
- Transparency: crystals are transparent to translucent.
- Crystal System is orthorhombic; mm2
- Crystal Habits include sprays of needle thin acicular crystals with a pyramidal termination. Also nodules, fiberous and massive crusts.
- Cleavage is perfect in two directions, prismatic. Cleavage is rarely seen due to small crystal size.
- Fracture is conchoidal.
- Hardness is 5 - 5.5.
- Specific Gravity is approximately 2.2 (very light)
- Streak is white.
- Associated Minerals are quartz, apophyllite, benitoite, heulandite, stilbite and other zeolites.
- Notable Occurrences include Poona, India; San Benito, California; New Jersey and Nova Scotia.
- Best Field Indicators are crystal habit, density and associations.