THE MINERAL SCOLECITE
THE MINERAL SCOLECITE
- Chemistry: CaAl2Si3O10-3H2O, Hydrated calcium aluminum silicate
- Class: Silicates
- Subclass: Tectosilicates
- Group: Zeolites
- Uses: mineral specimen and chemical filter
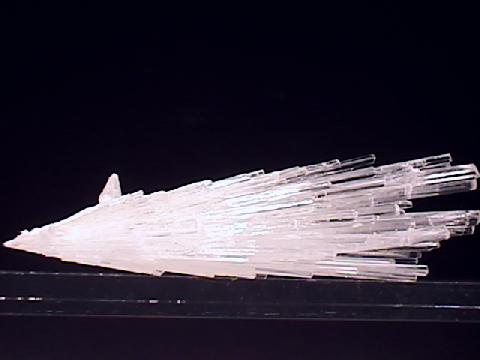
Specimens
Scolecite's structure has a typical zeolite openness about it that allows large ions and molecules to reside and actually move around inside the overall framework. The structure contains open channels that allow water and large ions to travel into and out of the crystal structure. The size of these channels controls the size of the molecules or ions and therefore a zeolite like scolecite can act as a chemical sieve. Scolecite's structure contains aligned chains of silicate tetrahedrons. This produces the needle-like crystals and the cleavage results from the weaker bonds between the chains.
Scolecite, a calcium zeolite, natrolite, a sodium zeolite, and mesolite, a calcium and sodium zeolite, are closely related and sometimes found together. The presence of calcium in two of the minerals slightly alters the structure from that of natrolite; from an orthorhombic symmetry to a monoclinic symmetry. However, twinning of scolecite and mesolite often make them look orthorhombic All three minerals are referred to as "chain" or "needle" zeolites. They are similar and hard to distinguish when in clusters with radiating, acicular habits. Natrolite tends to forms thin crystals with pyramidal terminations, but mesolite's fibrous crystals are usually the thinnest crystals of the three minerals. Scolecite's larger crystals tend to be more robust and durable. These characteristics are only generalities and can not be used as dependable identifying traits. Absolute identification can not be made by ordinary means.
PHYSICAL CHARACTERISTICS:
- Color is clear or white.
- Luster is vitreous to silky.
- Transparency: crystals are transparent to translucent.
- Crystal System is monoclinic; 2/m
- Crystal Habits include sprays of thin acicular crystals with slanted terminations. Also occurs in radiating fibrous clusters.
- Cleavage is perfect in two directions, prismatic. Cleavage is hard to see due to small crystal size.
- Fracture is conchoidal.
- Hardness is 5.
- Specific Gravity is approximately 2.2 (very light)
- Streak is white.
- Associated Minerals are quartz, apophyllite, babingtonite, heulandite, stilbite and other zeolites.
- Notable Occurrences include Poona, India; Riverside Co., California; Iceland; Skye Scotland and Santa Catarina, Brazil.
- Best Field Indicators are crystal habit, hardness, density and associations.