 THE
MINERAL ANALCIME
THE
MINERAL ANALCIME
- Chemistry: NaAlSi2O6-H2O, Hydrated Sodium Aluminum Silicate
- Class: Silicates
- Subclass: Tectosilicates
- Group: Zeolites
- Uses: mineral specimen and chemical filter.
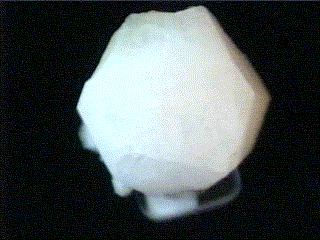
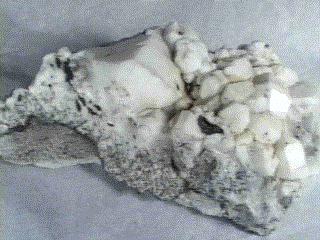
Specimens
Analcime is a popular and interesting mineral. It is sometimes known
as analcite, although analcime is preferred. It is one of the few minerals
that characteristically forms its own unique crystal. Well, not quite its
own unique crystal, but pretty close. It forms the isometric
Distinguishing analcime from the garnets and leucite is relatively easy in some cases. The garnets are much harder and usually deeply colored. Leucite has a much lower density and usually has a dull luster. Also leucite is typically embedded in host rock where as analcime, when displaying good crystals and not massive or granular, is loose or attacted to other minerals in volcanic cavities.
Analcime, AlSi2O6-H2O is actually distantly related to leucite, KAlSi2O6. Leucite is a member of the feldspathoid group of minerals. Minerals whose chemistries are close to that of the alkali feldspars but are poor in silica (SiO2) content, are called feldspathoids. Feldspathoids are commonly found in silica-poor igneous rocks, where analcime is sometimes present as well. Analcime is sometimes placed in the feldspathoid group since its chemistry and occassional occurrences are similar.
Analcime's structure however has a typical zeolite openness about it that allows large ions and molecules to reside and actually move around inside the overall framework. The structure contains large open channels that allow water and large ions to travel into and out of the crystal structure. The size of these channels controls the size of the molecules or ions, and therefore a zeolite like analcime can act as a chemical sieve.
PHYSICAL CHARACTERISTICS:
- Color is clear, white or gray, with greenish, yellowish and reddish tints possible.
- Luster is vitreous.
- Transparency: crystals are transparent to translucent.
- Crystal System is isometric; 4/m bar 3 2/m
- Crystal Habits include the characteristic trapezohedron as well as the rare cube modified by trapezohedral faces. Also granular and massive.
- Cleavage is absent.
- Fracture is uneven.
- Hardness is 5 - 5.5.
- Specific Gravity is approximately 3.2 (average)
- Streak is white.
- Associated Minerals are quartz, calcite, serandite, apophyllite, natrolite, stilbite, heulandite and other zeolites.
- Notable Occurrences include Mont St. Hilaire, Quebec; Iceland; several localities in Oregon, Colorado and New Jersey, USA; Nova Scotia and Switzerland.
- Best Field Indicators are crystal habit, density, low hardness, luster and associations.