The Mineral SERANDITE
The Mineral SERANDITE
- Chemistry: Na(Mn, Ca)2Si3O8(OH), Sodium Manganese Calcium Silicate Hydroxide
- Class: Silicates
- Subclass: Inosilicates
- Uses: Only as mineral specimens.
Specimens
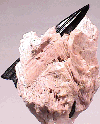
Serandite forms a series with the mineral pectolite, NaCa2Si3O8(OH). The structures are similar but serandite has a significant percentage of manganese ions which substitute for a portion of the calciums in the pectolite. It is the manganese ions which cause the pink color of serandite. Several other manganese minerals have a distinct pink color such as the carbonate rhodochrosite and the silicates rhodonite and inesite. However serandite's color is more of a salmon pink the these pink minerals and it has a silky luster that is distinct.
PHYSICAL CHARACTERISTICS:
- Color is pink to reddish pink and off white.
- Luster is vitreous to silky.
- Transparency crystals are transparent to translucent.
- Crystal System is triclinic; bar 1
- Crystal Habits include prismatic crystals with a distorted hexagonal cross-section. Also massive and compact.
- Cleavage is perfect in two directions at close to right angles.
- Fracture is splintery due to the cleavage.
- Hardness is 4.5
- Specific Gravity is approximately 3.2 - 3.4 (above average for translucent)
- Streak is white.
- Associated Minerals include calcite, analcime, vesuvianite, grossular garnetand many extremely rare minerals.
- Notable Occurrences include the famous mines at St. Hilaire, Quebec, Canada and Roma Island, Los Islands, Guinea.
- Best Field Indicators are crystal habit, color, luster, associations, locality and cleavage.