 |
and other properties such as
|
Parting in Minerals
Parting is a property that often gets confused with cleavage. And there is good reason for that. Parting is a break along structural planes and is parallel to a possible face, just like cleavage. However, parting differs from cleavage in some important ways. It cannot be found in every specimen as is true of cleavage for most every cleavable mineral. It is not absolutely repeatable or reproducible as is cleavage down to theoretically the very atomic layers that cause cleavage.
Parting is caused by pressures that are applied to a crystal or by twinning. The pressure breaks the crystal on a plane of weakness. The plane between twins in a crystal can also break and form a parting plane, but this has been disputed and is at best inconsistent as some twin planes may actually be a very strong suture-like planes resistant to breakage. With pressure parting, the actual break formed long before the crystal was excavated from the ground and may be due to tectonic or isostatic pressures that have been forced on the crystal.
Most parting is seen as fracture lines that are incomplete or healed (the crystal continued to grow and sealed the break) and might appear as
striations or planes of concentrated
inclusions.
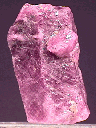
The picture above is of a
ruby crystal with rhombohedral parting appearing as thin, criss-crossing, pale colored planes (note how they parallel the "face" at the top).
As stated above, parting is not a consistent property, but when it is seen it can be an aid in identification.
Some mineralogists have suggested that parting is nothing more than just very poor cleavage and there is some evidence to support that.
The Reaction to Acids
An important property in minerals is how they react to acids.
All the minerals that have some reaction
to acids tend to be carbonates
and a few other minerals that contain significant amounts of carbonate ions.
The reaction is written as follows for
calcite,
the mineral for whom this test is made famous:
The carbon dioxide gas (CO2) is given off as bubbles and the calcium simply dissolves in the left over water. The bubbles or effervescence is the reaction we are looking for and indicates the presence of carbonate ions. Two hydrogens attack the carbonate ion (CO3) and detach one oxygen leaving the CO2 gas. The hydrogens are supplied by the acid which is generally dilute hydrochloric acid (<10% solution) or dilute acetic acid (vinegar). Other acids can be used with similar results but these two acids are the most user friendly as they are relatively mild. The key to this property is not the fact that the mineral reacts, although that is diagnostic, but how well it reacts. With calcite and aragonite, the two most common carbonate minerals, the reaction to a cold, dilute acid is easily accomplished and is often so energetic that it is associated with a fizzing noise. For dolomite and other carbonates the reaction is less vigorous and may only produce a small reaction when the mineral is powdered or the acid is heated. In some cases, the reaction is very slow and is only seen at all in the eventual dissolution of the mineral.
The Scent of a Mineral
Yes some minerals do have odors! In fact there are quite a few of them. Generally the odor is not strong unless the mineral has been struck or is freshly excavated. It is no surprise that the best known smelly mineral is sulfur. Other minerals that smell are often sulfides, arsenic based minerals, or clays. The sulfide mineral marcasite gives off a noticeable sulfur-like odor when it decomposes and the arsenic sulfide mineral arsenopyrite gives off a garlic smell when this mineral is struck or crushed. The smell in the sulfide minerals comes from the reaction of oxygen in the air to the sulfur, forming odious scented molecules such as sulfur dioxide (SO2). Arsenic minerals tend to have a garlic scent as is consistent with the element and poison arsenic. The clay minerals have, for lack of a better term, a clay-like smell that is sometimes called earthy. Occasionally a mineral will have a rather distinct and therefore diagnostic odor, but this is a fairly uncommon property and not reliable in general.
Minerals Have Feelings Too
Minerals that have a distinctive feel are generally low in hardness, but not always. Several metallic minerals such as copper have a jagged feel due to minute, sharp protrusions. Minerals such as molybdenite, graphite, serpentine, talc and several other clay minerals have a greasy or oily feel. At times feel can distinguish two minerals, but because feel is so subjective it is impossible to tell a person how a mineral should feel in comparison to another. Quite literally, hands on experience with these minerals is the only way to learn their characteristic "feels".
The Solubility of Minerals
Minerals do dissolve. In very idealistic terms, pure water can dissolve any mineral at least partially. Water is known as the "universal solvent", after all. However most minerals do not dissolve in water to any great extent that we can detect in a short period of time and without quantitative chemical tests. The ones that do dissolve rapidly are therefore considered soluble and this can be diagnostic. Most soluble minerals are nitrates, borates, some carbonates, some sulfates and some phosphates as well as some organic minerals. At times the degree to which a mineral is soluble is important as there are several closely related minerals (such as parisite and its relatives) that dissolve with much different speeds making for easier identification.
Solubility is not just limited to water. Many acids are capable of dissolving minerals where water has no effect. Carbonates are especially susceptible to acids (see reaction to acids). Some metals, even gold, can be dissolved in a mixture of one part nitric acid and three to four parts hydrochloric acid called "aqua regia". Of course, identification of minerals using solubility is a test that could destroy the specimen, if it is soluble. An unneeded fragment can be used for such tests.
Inclusions in Minerals
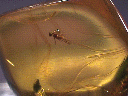
INSECT INCLUSION
IN AMBER
Many minerals have crystals of other minerals, air, water, tar, petroleum, rocks and (in the case of amber) even animals included in their interiors. They are called, appropriately enough, inclusions. These inclusions are sometimes accidental such as when one crystal was growing and another mineral begins to make a small crystal on the surface of the earlier mineral. The first mineral continues to grow and may grow over and around the second mineral, thereby enclosing it in its crystal.
The second type of inclusion involves minerals that formed after initial crystallization and is a result of exsolution. Some chemistries are favorable at certain temperatures and pressures, but are unstable at different temperatures and pressures. The minerals will then try and convert to a more stable chemistry and this often leads to the fractioning out of undesirable chemistries, ie different minerals. Rutile, TiO2, is a common inclusion mineral that forms in this way. Rutile inclusions are responsible for the effects of asterism and chatoyancy.
Some very rare minerals are only known as small inclusions in other minerals.
Inclusions of air and water are called two phase inclusions and are commonly found in gypsum and quartz. Identification of inclusions is difficult because few property tests (generally limited to color, translucency, luster and maybe crystal habit) can be performed on the including crystal without removing it from the host mineral. Some optic tests can be performed however and a reliable analysis can usually be obtained by a laboratory. Some invaluable and historic gemstones contain inclusions that were identified in this way. Some inclusions turned out to be other gemstone minerals! At times inclusions can be diagnostic and even assist in the identification of the minerals locality. Emeralds mined in Russia for instance, are known to have tiny inclusions of actinolite, unlike other emeralds.
