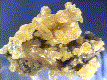
 NATIVE SULFUR
NATIVE SULFUR
- Chemistry: S , Elemental Sulfur
- CLASS: Elements
- Uses: Major ore of sulfur which is used for chemical production
Specimens
Many people don't like the smell of sulfur, which is not quite accurate, as sulfur itself has essentially no scent. As for the odor we detect, this occurs when water (including humidity in the air) mixes with the sulfur and a small amount of hydrogen sulfide (H2S) gas is produced. Although only small amounts of H2S form this way, it is a powerful odor producer and is the dominating contributor to the odor of rotten eggs. Rest assured, though, that most specimens of sulfur, when kept dry, do not emit a strong odor and this is not difficult for collectors of fine sulfur specimens to insure.
Sulfur easily burns in air with a blue flame, releasing the oxide which not only has a strong, distinctive odor (associated with brimstone, an ancient name for sulfur), but the sulfur diode gas reacts with moisture in the lungs and nasal passages, turning into toxic sulfurous acid.
Sulfur melts easily at a temperature only slightly above the boiling point of water. The color of molten sulfur is yellow at relatively cool temperatures (116 degrees Celsius or 240 degrees Fahrenheit), but gradually turns blood-red as it is heated to 200 degrees C / 400 degrees F, by which point the initially runny liquid has thickened due to polymerization.
Note that sulfur is quite brittle, and handling should be minimized. Rapid temperature changes can cause cracking - even holding a cool specimen in a warm hand may break it.
PHYSICAL CHARACTERISTICS:
- Color is a strong yellow color in thick crystals and duller yellow in small crystals to pale yellow in massive or powdery forms. Can also be reddish or greenish yellow with impurities.
- Luster is vitreous to more often resinous or earthy in massive forms.
- Transparency is transparent to translucent.
- Crystal System is orthorhombic; 2/m 2/m 2/m
- Crystal Habits include mostly massive or powdery forms but well shaped blocky crystals are common. Crystals can be made up of two dipyramids, one with steeper faces than the other, prisms and/or pinacoids in various combinations.
- Cleavage is very poor in two directions.
- Fracture is conchoidal.
- Streak is yellow.
- Hardness is 2.
- Specific Gravity is 2.0 - 2.1 (well below average)
- Associated Minerals are celestite, calcite, aragonite and gypsum.
- Other Characteristics: odor (see above), poor heat conductivity makes it brittle when heated and can actually crack if held tightly in a person's hand.
- Notable Occurrences include Michigan and Ohio, USA; Sicily; Poland and Chile.
- Best Field Indicators are color, odor, heat sensitivity, lack of good cleavage and crystal habit.
 Amethyst Galleries' Mineral Gallery MINERALS |

$ 90.00

$ 40.00

sul-4 ($ 40.00)

$ 30.00

sul-6 ($ 30.00)

$ 30.00

sul-7 ($ 30.00)

$ 75.00

sul-8 ($ 75.00)

$ 23.00


sul-9 ($ 23.00)

$ 90.00


sul-10 ($ 90.00)

$ 130.00


sul-11 ($130.00)

$ 285.00



$ 37.00
sul-13 ($ 37.00)

$ 35.00

sul-14 ($ 35.00)

$ 140.00


sul-15 ($140.00)

$ 40.00

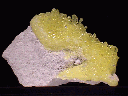
sul-16 ($ 40.00)

$ 38.00

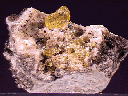
sul-17 ($ 38.00)

$ 50.00

sul-18 ($ 50.00)

$ 95.00

sul-19 ($ 95.00)

$ 50.00

sul-20 ($ 50.00)

$ 28.00

sul-21 ($ 28.00)

$ 36.00


sul-22 ($ 36.00)

$ 27.00

sul-23 ($ 27.00)

$ 28.00

sul-24 ($ 28.00)

$ 23.00

sul-25 ($ 23.00)

$ 25.00

sul-26 ($ 25.00)

$ 36.00

sul-27 ($ 36.00)

$ 38.00

sul-28 ($ 38.00)

$ 75.00


sul-29 ($ 75.00)

$ 60.00


sul-30 ($ 60.00)

$ 50.00


sul-31 ($ 50.00)

$ 25.00

sul-32 ($ 25.00)

$ 115.00



$ 45.00


sul-34 ($ 45.00)

$ 58.00


sul-35 ($ 58.00)

$ 135.00


sul-36 ($135.00)

$ 75.00


sul-37 ($ 75.00)