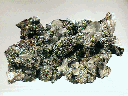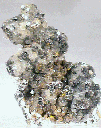The Mineral CALCITE




- Chemistry: CaCO3, Calcium Carbonate
- Class: Carbonates
- Group: Calcite
- Uses: In cements and mortars, production of lime, limestone is used in the steel industry; glass industry, ornamental stone, chemical and optical uses and as mineral specimens.
- Calcite's Physical Properties
-
Specimens
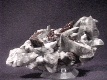
With calcite so abundant and so widely distributed it is no wonder that it can be so varied. The crystals of calcite can form literally a thousand different shapes by combining the basic forms of the positive rhombohedron, negative rhombohedron, steeply, moderately and slightly inclined rhombohedrons, various scalahedrons, prism and pinacoid to name a few of the more common forms. There are more than 300 crystal forms identified in calcite and these forms can combine to produce the thousand different crystal variations. Calcite also produces many twin varieties that are favorites among twin collectors. There are also phantoms, included crystals, color varieties, pseudomorphs and unique associations. There simply is no end to the varieties of calcite.
There are several varieties of calcite and it would be impossible to
describe them all. However there are a few standouts. Possibly the most
well known of calcite's varieties is its most common form, the classic
scalenohedron
or "Dogtooth Spar" as it is sometimes called. This variety
appears as a double pyramid or
dipyramid,
but is actually a distinctly different form. The point of the scalenohedron
is sharp and resembles the canine tooth of a dog, hence the name. Beautiful
clear colorless or amber-orange examples of this variety are considered
classics and outstanding examples come from Pugh Quarry, Ohio;
Not necessarily a variety of calcite, cave formations are certainly a unique aspect of calcite's story. Calcite is the primary mineral component in cave formations. Stalactites and stalagmites, cave veils, cave pearls, "soda straws" and the many other different cave formations that millions of visitors to underground caverns enjoy are made of calcite. It is the fact that calcite is readily dissolved that these formations occur. Overlying limestones or marbles are dissolved away by years and years of slightly acidic ground water to percolate into the caverns below. In fact the caverns themselves may have been the result of water dissolving away the calcite rich rock. As the calcite enriched water enters a relatively dry cavern, the water starts to evaporate and thus precipitate the calcite. The resulting accumulations of calcite are generally extremely pure and are colored if at all, by very small amounts of iron or other impurities.
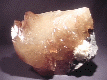
Mexican onyx is a variety of calcite that is used extensively for ornamental purposes. It is carved into figurines and is so popular that almost every child in the USA owns a small onyx animal or two. Carvings such as vases, bookends, plates, eggs, obilisks, pyramids and statues are all popular. It is not the same onyx as the quartz variety of onyx which is a little more precious (it is used in jewelry) and is banded white and black. To avoid confusion it is best to refer to it as Mexican Onyx. Mexican onyx is banded with multiple orange, yellow, red, tan, brown and white colors that have marble-like texture. The carvings are quite attractive and affordable; a rare combination!
Another variety is the so called "Iceland Spar", which is basically clear cleaved fragments of completely colorless (ice-like) calcite. Originally discovered and named after Eskifjord, Iceland where the calcite is found in basalt cavities. In rock shops around the world, iceland spar is available in large quantities and at affordable prices and are popular among children. Most of today's iceland spar comes from Mexico. The iceland spar displays the classic cleavage form of calcite, the rhombohedron. Iceland spar was and is used for optical equipment and during World War II it was a strategic mineral as it was used for the sighting equipment of bombardiers and gunners. It is iceland spar that best demonstrates the unique property of calcite called double refraction.
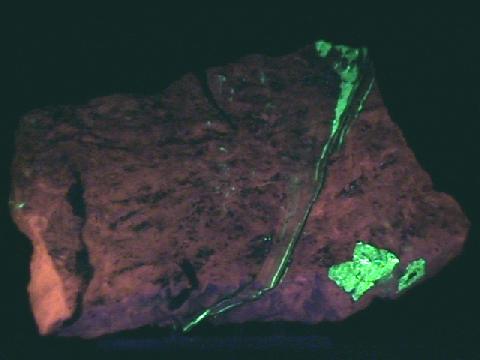
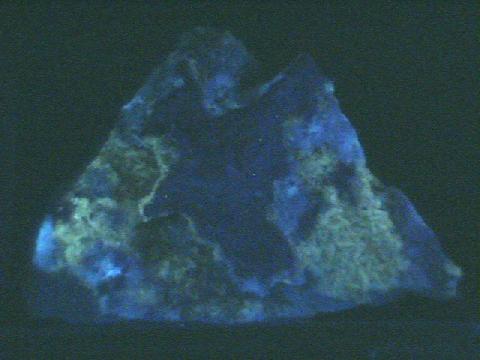
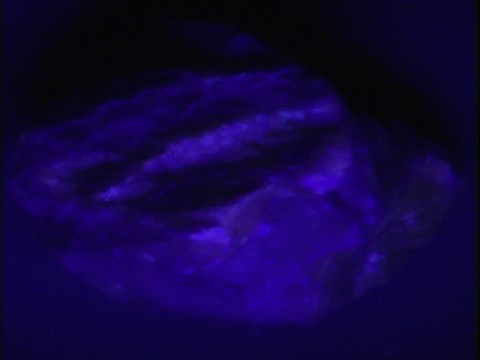
Fluorescence,
phosphorescence,
thermoluminescence
and
The best property of calcite is the acid test.
Why? Because
calcite always will effervesce (bubble) when even cold weak acids are placed
on specimens. Even the cement in sandstones will effervesce assuring the
geologist of identification of the cementing mineral. The reason for the
bubbling is in the formula below:
The carbon dioxide gas (CO2)
is given off as bubbles and the calcium dissolves in the residual water.
Any acid, just about, can produce these results, but dilute hydrochloric
acid or vinegar are the two recommended acids for this test. Other carbonates
such as dolomite
or siderite
do not react as easily with these acids as does calcite and this leads
to differentiating these somewhat similar minerals more readily.
Calcite is intricately tied to carbon dioxide in another way. Since many sea organisms such as corals, algae and diatoms make their shells out of calcite, they pull carbon dioxide from the sea water to accomplish this in a near reverse of the reaction above. This is fortuitous for us, as carbon dioxide has been found to be a green house gas and contributes to the so called "green house gas effect". Environmentally then, calcite is very important and may have been quite important to the successful development of our planet in the past. By pulling carbon dioxide out of the sea water, this biological activity allows more of the carbon dioxide in the air to dissolve in the sea water and thus acts as a carbon dioxide filter for he planet. Environmentalists are now actively engaged in determining if this activity can be increase by human intervention to the point of warding off the "green house gas effect". A significant amount of calcite precipitation in sea water is undoubtedly inorganic, but the exact amount that this contributes is not well known. Calcite and other carbonate minerals are very important minerals in the ocean ecosystems of the world.
Calcite is not the only calcium carbonate mineral. There are no less
than three minerals or phases of CaCO3.
Aragonite
and
Calcite is truly one of the best collection type minerals. There are lots of interesting forms and varieties as well as colorful and beautiful specimens to collect. It is generally easy to identify using its rhomohedral cleavage, reaction to acid and double refraction and makes for a great classroom example of these properties. If it is not the significant mineral on a specimen, it might be an accessory to other wonderful minerals and only enhancing their attractiveness. With its many different forms, environments, associations and colors, a collector could never have all possible combinations of calcite covered.
PHYSICAL CHARACTERISTICS:
- Color is extremely variable but generally white or colorless or with light shades of yellow, orange, blue, pink, red, brown, green, black and gray. Occasionally iridescent.
- Luster is vitreous to resinous to dull in massive forms.
- Transparency: Crystals are transparent to translucent.
- Crystal System is trigonal; bar 3 2/m
- Crystal Habits are extremely variable with almost any trigonal form possible. Common among calcite crystals are the scalenohedron, rhombohedron, hexagonal prism, and pinacoid. Combinations of these and over three hundred other forms can make a multitude of crystal shapes, but always trigonal or pseudo-hexagonal. Twinning is often seen and results in crystals with blocky chevrons, right angled prisms, heart shapes or dipyramidal shapes. A notch in the middle of a doubly terminated scalenohedron is a sure sign of a twinned crystal. lamellar twinning also seen resulting in striated cleavage surfaces. Pseudomorphs after many minerals are known, but easily identified as calcite. Also massive, fibrous, concretionary, stalactitic, nodular, oolitic, stellate, dendritic, granular, layered, etc. etc.
- Cleavage is perfect in three directions, forming rhombohedrons.
- Fracture is conchoidal.
- Hardness is 3 (only on the basal pinacoidal faces, calcite has a hardness of less than 2.5 and can be scratched by a fingernail).
- Specific Gravity is approximately 2.7 (average)
- Streak is white.
- Other Characteristics: refractive indices of 1.49 and 1.66 causing a significant double refraction effect (when a clear crystal is placed on a single line, two lines can then be observed), effervesces easily with dilute acids and may be fluorescent, phosphorescent, thermoluminescence and triboluminescent.
- Associated Minerals are numerous but include these classic associations: Fluorite, quartz, barite, sphalerite, galena, celestite, sulfur, gold, copper, emerald, apatite, biotite, zeolites, several metal sulfides, other carbonates and borates and many other minerals.
-
Notable Occurrences include Pugh Quarry, Ohio; Rosiclare, Illinois;
Franklin,
New Jersey; Elmwood, Tennessee; Brush Creek and other Missouri,
Wisconsin, Kansas and Oklahoma localities, USA; Andreasburg, Harz
Mountains
and Saxony, Germany; Brazil; Guanajuato, Mexico;
Cornwall, Durham and Lancashire, England; Bombay area of India; Eskifjord, Iceland; many African localities as well as others around the world with their own unique varieties. - Best Field Indicators are crystal habit, reaction to acid, abundance, hardness, double refraction and especially cleavage.