THE
MINERAL TRONA
THE
MINERAL TRONA
- Chemistry: Na3(HCO3)(CO3) - 2H2O , Hydrated Sodium Bicarbonate Carbonate.
- Class: Carbonates
- Uses: Only as mineral specimens.
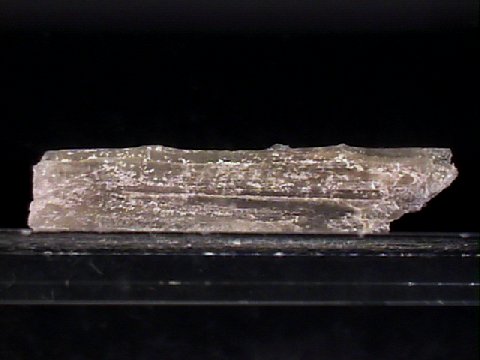
Specimens
Trona is the type mineral so-to-speak for several sodium carbonates that form in non-marine evaporite deposits.
Other sodium carbonates include
gaylussite,
PHYSICAL CHARACTERISTICS:
- Color is gray, colorless, white, pale brown or yellowish.
- Luster is vitreous.
- Transparency: Crystals are transparent to translucent.
- Crystal System is monoclinic; 2/m.
- Crystal Habits include prismatic to blocky crystals, but usually massive, fibrous or columnar.
- Cleavage is perfect in one direction and poor in two others.
- Fracture is subconchoidal to uneven.
- Hardness is 2.5 - 3.
- Specific Gravity is 2.1 (well below average)
- Streak is white.
- Other Characteristics: Has an alkaline taste.
- Associated Minerals include
hanksite,
gaylussite,
halite,
pirssonite,
northupite , nahcolite, borax and calcite. - Notable Occurrences include Searles Lake, San Bernardino County; Borax Lake, Lake County; Owens Lake, Inyo County and Mono Lake, Mono County, California and Green River, Wyoming, USA; Iran; Tibet; Mont Saint-Hilaire, Quebec, Canada and Mongolia.
- Best Field Indicators: environment of formation, color, cleavage, density, crystal habit, taste and locality.