
 THE MINERAL PYRITE
THE MINERAL PYRITE
- Chemistry: FeS2, Iron Sulfide
- Class: Sulfides
- Group: Pyrite
- Uses: A very minor ore of sulfur for sulfuric acid, used in jewelry under the trade name "marcasite" and as mineral specimens.
Specimens
Pyrite is a polymorph of marcasite, which means that it has the same chemistry, FeS2, as marcasite; but a different structure and therefore different symmetry and crystal shapes. Pyrite is difficult to distinguish from marcasite when a lack of clear indicators exists.
Pyrite's structure is analogous to galena's structure with a formula of PbS. Galena, though, has a higher symmetry. The difference between the two structures is that the single sulfur of galena is replaced by a pair of sulfurs in pyrite. The sulfur pair are covalently bonded together in essentially an elemental bond. This pair disrupts the four fold symmetry that a single atom of sulfur would have preserved and thus gives pyrite a lower symmetry than galena.
Although pyrite is common and contains a high percentage of iron, it has never been used as a significant source of iron. Iron oxides such as hematite and magnetite, are the primary iron ores. Pyrite is not as ecomonical as these ores possibly due to their tendency to form larger concentrations of more easily mined material. Pyrite would be a potential source of iron if these ores should become scarce.
Pyrite has been mined for its sulfur content, however. During WWII, sulfur was in demand as a strategic chemical and North American native sulfur mines were drying up. A sulfide deposit near Ducktown, Tennessee contained commercially valuable deposits of pyrite and other sulfides such as pyrrhotite and pentlandite and produced the needed sulfur as well as iron and other metals. The sulfur was used in the production of sulfuric acid, an important chemical for industrial purposes. Now most sulfur production comes from H2S gas recovered from natural gas wells.
PHYSICAL CHARACTERISTICS:
- Color is brassy yellow.
- Luster is metallic.
- Transparency: Crystals are opaque.
- Crystal System is isometric; bar 3 2/m
- Crystal Habits include the cube, octahedron and pyritohedron (a dodecahedron with pentagonal faces) and crystals with combinations of these forms. Good interpenetration twins called iron crosses are rare. Pyrite is commonly found in nodules. A flattened nodular variety called "Pyrite Suns" or "Pyrite Dollars" is popular in rock shops. Also massive or reniform and replaces other minerals and fossils forming pseudomorphs or copies.
- Cleavage is very indistinct.
- Fracture is conchoidal.
- Hardness is 6 - 6.5
- Specific Gravity is approximately 5.1+ (heavier than average for metallic minerals)
- Streak is greenish black.
- Other Characteristics: Brittle, striations on cubic faces caused by crossing of pyritohedron with cube. (note - striations on cube faces also demonstrate pyrite's lower symmetry). Pyrite (unlike gold) is not malleable.
- Associated Minerals are quartz, calcite, gold, sphalerite, galena, fluorite and many other minerals. Pyrite is so common it may be quicker to name the unassociated minerals.
- Notable Occurrences include Illinois and Missouri, USA; Peru; Germany; Russia; Spain; and South Africa among many others.
- Best Field Indicators are crystal habit, hardness, streak, luster and brittleness.
 Amethyst Galleries' Mineral Gallery MINERALS |

$ 96.00

pyr-4 ($ 96.00)

$ 18.00
pyr-7 ($ 18.00)

$ 150.00

pyr-8 ($150.00)

$ 45.00


pyr-15 ($ 45.00)

$ 60.00


pyr-18 ($ 60.00)

$ 23.00

pyr-21 ($ 23.00)

$ 24.00


pyr-23 ($ 24.00)

$ 95.00


pyr-25 ($ 95.00)

$ 21.00

pyr-27 ($ 21.00)

$ 25.00


pyr-28 ($ 25.00)

$ 25.00

pyr-29 ($ 25.00)

$ 27.00

pyr-30 ($ 27.00)

$ 30.00
pyr-31 ($ 30.00)

$ 35.00
pyr-32 ($ 35.00)

$ 35.00

pyr-33 ($ 35.00)

$ 65.00


pyr-34 ($ 65.00)

$ 36.00


pyr-36 ($ 36.00)

$ 350.00



$ 40.00

pyr-39 ($ 40.00)

$ 45.00

pyr-40 ($ 45.00)

$ 105.00

pyr-41 ($105.00)

$ 105.00

pyr-42 ($105.00)

$ 30.00


pyr-45 ($ 30.00)

$ 85.00

pyr-46 ($ 85.00)

$1500.00



$ 130.00


pyr-48 ($130.00)

$ 25.00

pyr-49 ($ 25.00)

$ 30.00

pyr-50 ($ 30.00)

$ 28.00

pyr-51 ($ 28.00)

$ 28.00

pyr-52 ($ 28.00)

$ 25.00


pyr-53 ($ 25.00)

$ 28.00

pyr-54 ($ 28.00)

$ 45.00

pyr-55 ($ 45.00)

$ 50.00

pyr-56 ($ 50.00)

$ 36.00

pyr-57 ($ 36.00)

$ 70.00

pyr-58 ($ 70.00)

$ 80.00

pyr-59 ($ 80.00)

$ 30.00

pyr-60 ($ 30.00)

$ 50.00

pyr-61 ($ 50.00)

$ 25.00

pyr-62 ($ 25.00)

$ 70.00

pyr-63 ($ 70.00)

$ 49.00

pyr-64 ($ 49.00)

$ 48.00

pyr-65 ($ 48.00)

$ 33.00

pyr-66 ($ 33.00)

$ 25.00

pyr-67 ($ 25.00)

$ 28.00

pyr-68 ($ 28.00)

$ 29.00

pyr-69 ($ 29.00)

$ 25.00

pyr-70 ($ 25.00)

$ 54.00

pyr-71 ($ 54.00)

$ 54.00

pyr-72 ($ 54.00)

$ 28.00

pyr-73 ($ 28.00)

$ 60.00

pyr-74 ($ 60.00)

$ 42.00

pyr-75 ($ 42.00)

$ 25.00

pyr-76 ($ 25.00)

$ 45.00

pyr-77 ($ 45.00)

$ 115.00


pyr-78 ($115.00)

$ 70.00


pyr-79 ($ 70.00)

$ 60.00
pyr-81 ($ 60.00)

$ 90.00

pyr-82 ($ 90.00)

$ 50.00


pyr-83 ($ 50.00)

$ 26.00

pyr-84 ($ 26.00)

$ 70.00


pyr-85 ($ 70.00)

$ 70.00


pyr-86 ($ 70.00)

$ 27.00
pyr-87 ($ 27.00)

$ 135.00


pyr-88 ($135.00)

$ 135.00


pyr-89 ($135.00)

$ 150.00

pyr-90 ($150.00)

$ 35.00

pyr-92 ($ 35.00)

$ 32.00

pyr-93 ($ 32.00)

$ 45.00

pyr-94 ($ 45.00)

$ 45.00

pyr-95 ($ 45.00)

$ 43.00

pyr-96 ($ 43.00)

$ 41.00

pyr-97 ($ 41.00)

$ 46.00

pyr-98 ($ 46.00)

$ 37.00

pyr-99 ($ 37.00)

$ 43.00

pyr-100 ($ 43.00)

$ 38.00

pyr-102 ($ 38.00)

$ 97.00


pyr-101 ($ 97.00)

$ 50.00


pyr-103 ($ 50.00)

$ 25.00

pyr-104 ($ 25.00)
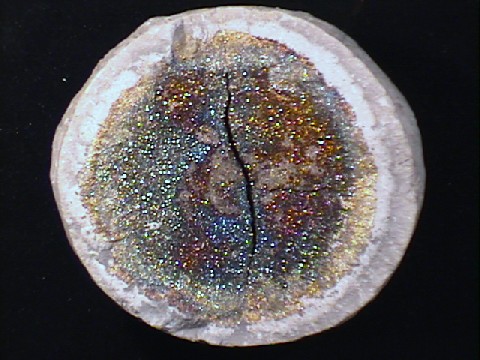
$ 95.00

pyr-105 ($ 95.00)

$ 50.00


pyr-108 ($ 50.00)

$ 60.00


pyr-109 ($ 60.00)

$ 30.00


pyr-106 ($ 30.00)

$ 35.00


pyr-107 ($ 35.00)