 THE MINERAL GALENA
THE MINERAL GALENA
Like:
Share:
- Chemistry: PbS, Lead Sulfide
- Class: Sulfides
- Group: Galena
- Uses: Major ore of lead and silver
Specimens
To quote Allen N Wollscheidt,
"Galena, back 75 years ago, was the stuff -- the crystal -- of crystal radio sets. Surely you have heard of these -- possibly your grand or greatgrandparents built radio receivers out of round Quaker Oats boxes, a few feet of copper wire, a pair of headphones and a little "cats-whisker-with crystal" gadget from the hardware store. Some worked so well, they could be heard across the room.Galena is a natural semiconductor and so the forerunner, the enabler, of all the electronic gadgets we have today, from telephones to TVs to GPS navigating systems as well as all sorts of medical equipment -- in short, modern life as we know it.”
PHYSICAL CHARACTERISTICS:
- Color is lead to silver gray sometimes with a bluish tint.
- Luster is metallic to dull in weathered faces.
- Transparency crystals are opaque.
- Crystal System is isometric; 4/m bar 3 2/m
- Crystal Habits include the cube, octahedron and combinations of the two. Spinel twinning is possible forming flattened crystals. Also massive and granular.
- Cleavage is perfect in four direction forming cubes.
- Fracture is uneven and rarely seen because of the perfect cleavage.
- Hardness is 2.5+
- Specific Gravity is approximately 7.5+ (heavy even for metallic minerals)
- Streak is lead gray
- Associated Minerals are calcite, dolomite, sphalerite, pyrite and other sulfide minerals, also lead oxidation minerals such as cerussite and anglesite.
- Other Characteristics: brighter metallic luster on cleavage surfaces than on crystal faces.
- Notable Occurances include Texas-Oklahoma-Missouri area, USA; Germany, Peru, Mexico, Zambia, and England.
- Best Field Indicators are crystal habit, cleavage and, perhaps most importantly, density.
 Amethyst Galleries' Mineral Gallery MINERALS |

GALENA specimen gal-1
$ 97.00
$ 97.00
Dims: 6" x 4.25" x 2"
Wt: 3.50lb
Pachapaqui Mine, Ancash, Peru
One of our largest specimens of Galena, this monstrosity has beautiful crystals up to 1 inch in diameter that show clean crystal form, frequent and interesting twinning formations, and in some spots, a beautiful irridescence caused by an oxide coating. Some of the crystals in this immense cluster are Sphalerites, with a darker, less metallic luster, and a different crystal form. A hive of small Quartz crystals grow around the cluster, and on its edges are a tiny smattering of Pyrites. This is a super-quality specimen that is just waiting for a collector.

gal-1 ($ 97.00)
Pachapaqui Mine, Ancash, Peru

GALENA specimen gal-4
$ 20.00
$ 20.00
Dims: 1.50" x 1.25" x 1"
Wt: 4.6oz
Naica, Mexico
What can I say? This specimen is neat! Here we have Galena in a variety of forms; I see hexagonal, square and triangular faces, along with large amounts of very fine layering that makes it look like cast metal in some places. Alongside the Galena are some fragments of Sphalerite crystals that are almost hard to tell apart from their host. To top it all off, there is a large crystal of clear Fluorite whose shape is difficult to define due to a crust of tiny Quartz crystals that covers it. This is really a beautiful and unique specimen.

gal-4 ($ 20.00)
Naica, Mexico

GALENA specimen gal-10
$ 48.00
$ 48.00
Dims: 4-1/2" x 3" x 2-1/2"
Wt: 2 lbs, 10 oz
Reynolds Co., Missouri, USA
This large hunk of galena is a partial crystal, but has several exceptional crystal faces that betray its form. It is a hybrid octahedron/cube according to the shapes of its faces, each of which have a remarkable texture due to visible growth patterns. Its underside has the look of classic, cleaved galena, although there does seem to be evidence of some partial healing in one area. There are also tiny calcite crystals scattered along its sides, and just a hint of chalcopyrite!

gal-10 ($ 48.00)
Reynolds Co., Missouri, USA

GALENA specimen gal-11
$ 27.00
$ 27.00
Dims: 2-3/4" x 2-3/8" x 2"
Wt: 11.1 oz
Pachapaqui Mine, Ancash, Peru
This Galena piece shows the pseudohexagonal formation that is so popular among collectors. The crystals look as if they've formed intergrowing hexagonal platelets. Although it's difficult to determine where one crystal ends and another begins, there is a particularly large (1/4 inch) crystal face exposed. Among these crystals is a light infestation of quartz crystals and a dusting of pyrite and sphalerite in one spot. I like this Galena specimen the best, due to the "mill-wheels"- it's quite impressive.


gal-11 ($ 27.00)
Pachapaqui Mine, Ancash, Peru

GALENA specimen gal-12
$ 50.00
$ 50.00
Dims: 5.0" x 4.8" x 2.4"(12.7 x 12.2 x 6.1 cm)
Wt: 2 lbs., 4.2 oz.(1.029 kg)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.
A few large, cubic Galena crystals rest on a bed of tiny ones mixed with dolomite and chalcopyrite crystals on this specimen. They are, of course, opaque and have the standard dark gray color and metallic luster of the mineral. All of the crystals are intergrown, creating a large, rectangular chunk; two of them are of nearly equal size and have grown slightly askew, so that their faces are almost but not quite parallel to each other. The tiny Galenas occur in the octahedral form with their points truncated by small secondary faces. The dolomite crystals occur as tiny white rhombohedrons, and the chalcopyrite crystals occur as disphenoid prisms. All of this rests on a large, dirty matrix of dolomitic limestone.

gal-12 ($ 50.00)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-13
$ 25.00
$ 25.00
Dims: 2.0" x 1.9" x 1.5"(5.1 x 4.8 x 3.8 cm)
Wt: 8.80 oz.(249.5 g)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.
The bulk of this specimen consists of a few intergrown Galena crystals. Like most Galena, these crystals are opaque, have a dark gray color and a metallic luster, and occur in the cubic form. They are all intergrown, and the two largest crystals are interpenetrating at a shallow angle to each other. Two well-formed but incomplete hexagonal prismatic calcite crystals span the distance between two perpendicular faces of the specimen, so that only their prisms are visible. A small amount of their dirty limestone matrix is visible, and contains small and misshapen Galena, calcite, and pyrite crystals. None of the Galena faces seem to be cleaved- I am pretty sure that this is the natural form of the crystals.


gal-13 ($ 25.00)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-14
$ 25.00
$ 25.00
Dims: 1.9" x 1.5" x 1.5"(4.8 x 3.8 x 3.8 cm)
Wt: 3.17 oz.(89.9 g)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.
This small hand specimen consists of a single cubic Galena crystal on a bed of dolomite. The cube is rather small, measuring 0.9 x 0.8 x 0.7"(2.3 x 2.0 x 1.8 cm), and is thus not shaped like a perfect cube. Its corners have been truncated by small secondary faces, and there is some noticeable damage to one of them, along with part of an adjacent edge. The crystal has good form, however, and striations on its faces that run at 45- and 90-degrees to each other. It has the standard dark gray color, metallic luster, and opacity of its kind. The base is made up almost entirely of Dolomite but contains a few badly-shaped Galenas.

gal-14 ($ 25.00)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-15
$ 30.00
$ 30.00
Dims: 2.3" x 1.7" x 1.5"(5.8 x 4.3 x 3.8 cm)
Wt: 7.89 oz.(223.9 g)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.
This specimen is dominated by an oblong pair of interpenetrating Galena crystals. They have the dark gray color, metallic luster and complete opacity of the mineral. Only a few shallow angles between separate faces betray the intergrowths of the 2 cubic crystals. The "unit" that they create measures roughly 1.6 x 1.2 x 1.0"(4.1 x 3.0 x 2.5 cm). It is attached to a small amount of dirty dolomitic host rock that is coated with dolomite crystals in one area and smaller, cleaved Galena crystals in another. These smaller Galenas are in turn coated with a crystalline, almost botryoidal chalcopyrite crust.

gal-15 ($ 30.00)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-16
$ 45.00
$ 45.00
Dims: 2.1" x 1.9" x 1.4"(5.3 x 4.8 x 3.5 cm)
Wt: 4.05 oz.(114.9 g)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.
Several small cubic Galena crystals are gathered together in a cluster on this specimen. They all possess the standard characteristics of Galena, i.e. dark gray color, metallic luster, high density, and opacity. Their form is very good, with cubic growth patterns visible on their faces and small secondary faces truncating their corners. They range in size from 0.1 - 0.6"(0.3 - 1.5 cm) square, and do not necessarily form perfect cubes. A few of them are incomplete, but none show any damage. They rest on a base that is made up primarily of tiny, white dolomite crystals, botryoidal chalcopyrite clusters, and smaller Galena cubes, most of which are incomplete.

gal-16 ($ 45.00)
Sweetwater Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-17
$ 50.00
$ 50.00
Dims: 2.1" x 1.9" x 1.6"(5.3 x 4.8 x 4.1 cm)
Wt: 6.55 oz.(185.7 g)
Naica, Chihuahua, Mexico
The Galena crystals that make up the bulk of this specimen are some of the most intriguing that I have seen. Though their forms are somewhat rounded, there are definite tetrahedral and cubo-octahedral shapes. Some of these shapes are malformed; a lot of this malformation has to do with interpenetration twinning, of which there are many examples on this specimen. There are also all sorts of growth-related striations and patterns on the faces and edges of each crystal. All have the gray color and dull metallic luster that is common in Galena. The largest of these crystals is a cubo-octahedron with one complete face exposed that measures 0.7" (1.8 cm) along each edge. There is a "filler" of white crystalline calcite between the Galenas, along with some heavily fractured sphalerite and even a few sparkly nodules of pyrite or marcasite. A small wooden "elbow" has been hot-glued to the specimen so that it will stand up for better display. I have always liked this specimen because of its odd forms and the patterning on its faces.


gal-17 ($ 50.00)
Naica, Chihuahua, Mexico

GALENA specimen gal-18
$ 25.00
$ 25.00
Dims: 3.3" x 2.4" x 1.0" (8.4 x 6.1 x 2.5 cm)
Wt: 8.32 oz.(235.9 g)
Naica, Chihuahua, Mexico
It is rather difficult to describe the Galena that makes up the bulk of this specimen, because I have not seen it look like this before. It occurs in what appears to be a heavily intergrown cluster of very rounded crystals that almost appear to have been partially melted. I am not sure, but maybe their rounded, almost botryoidal shape is due to water wear. The cluster has the standard metallic luster of Galena, but has a slightly brighter gray coloration than I am used to seeing, especially on undamaged crystal faces. Small, rounded clusters of dull white calcite partially cover the Galena- some of these clusters contain crystals of deep-yellow chalcopyrite. There is a single anhydrite crystal that is completely visible on one edge of the specimen. It measures almost exactly 1" (2.5 cm) long and is a milky white in color with a faint hint of blue. The specimen's underside, where it was broken off of its place of origin, shows what appears to be a core of sphalerite, around which the Galena grew. It has a black color with brown-red highlights and a bright, nearly adamantine luster. It is one of the more unusual Galena specimens that I have seen.

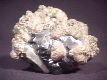
gal-18 ($ 25.00)
Naica, Chihuahua, Mexico

GALENA specimen gal-19
$ 22.00
$ 22.00
Dims: 3.0" x 2.0" x 2.0" (7.6 x 5.1 x 5.1 cm)
Wt: 10.7 oz. (304 g)
Amax Mine, Reynolds County, Missouri, U.S.A.
This lovely hand specimen consists of hundreds of intergrown Galena crystals. Most of these are tiny, not measuring more than 0.1" (3 mm) in diameter, but at least 20 of them exceed 0.3" (8 mm) in all dimensions, and the largest has dimensions of 0.6 x 0.5 x 0.5" (1.5 x 1.3 x 1.3 mm). All are in excellent condition, showing only a small amount of damage on the piece, and they all have very good octahedral form with their tips often truncated by small cube-oriented faces. They have the standard dark-gray color, dull metallic luster, and opacity of Galena, and are dusted with a thin layer of superfine pyrite or chalcopyrite, giving some of the crystals a dull golden appearance. There is no host rock or other material on the specimen.


gal-19 ($ 22.00)
Amax Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-21
$ 36.00
$ 36.00
Dims: 2.9" x 1.7" x 1.4" (7.4 x 4.3 x 3.6 cm)
Wt: 9.75 oz. (276.4 g)
Reynolds County, Missouri, U.S.A.
This small hand specimen consists of a crust made up of several intergrown Galena crystals. Though incomplete due to their intergrowth and the specimen's separation from its place of origin, one can see that the crystals have a definite octahedral form. Their surfaces are uneven due to large growth patterns on their surfaces, but their form is good. The largest of these crystals measures 1" (2.5 cm) along its only complete edge. All show a dull metallic luster, but their coloration is quite unusual and beautiful. The crystals' surfaces are likely coated with a thin layer of either a metallic oxide or another sulfide, as all have a colorful and intense iridescence that ranges from green to blue to violet. The surfaces that were formed by the specimen's separation have the standard dark gray coloration and a brighter metallic luster than those of the crystals, and bear a small amount of a dull, brownish host rock.


gal-21 ($ 36.00)
Reynolds County, Missouri, U.S.A.

GALENA specimen gal-22
$ 27.00
$ 27.00
Dims: 3.4" x 2.6" x 1.7" (8.6 x 6.6 x 4.3 cm)
Wt: 9.52 oz. (270.1 g)
Naica, Chihuahua, Mexico
This hand specimen consists mostly of a large but indeterminate number of intergrown Galena crystals. These crystals have what appears to be a cubo-octahedral form, but their intergrowth is so intense that most of their shapes are nearly indefinable. There is only a small amount of damage visible along the edges of the Galena formation, and all of the "crystals" have a moderately dark gray color and a rather dull metallic luster. Portions of this formation are covered with an extremely thin coating of a dull brownish-gold material that is likely chalcopyrite. There are several black sphalerite crystals among the Galena formation, which dominates one side of the piece. The other side of the piece is dominated by a formation of heavily-intergrown chalcopyrites that do not exceed 0.1" (3 mm). They have the standard deep golden color and metallic luster of chalcopyrite, and are also heavily intergrown.

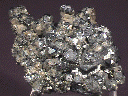
gal-22 ($ 27.00)
Naica, Chihuahua, Mexico

$ 150.00
Dims: 3.1 x 2.8 x 2.6" (7.9 x 7.1 x 6.6 cm)
Wt: 14.8 oz. (419.0 g)
level 3, Manto Calavera, Los Remedios Mine, Taxco, Guerrerro, Mexico
This hand specimen is made up mostly of intergrown pseudohexagonal Galena blades. It is difficult to assess their size due to their intense intergrowth, but I believe that they do not exceed 0.5" (1.3 cm) in diameter. They are generally in good condition, though there are several areas of visible, fresh damage. Aside from their intergrowth, all have good form, with well-defined edges and clean faces that possess a


level 3, Manto Calavera, Los Remedios Mine, Taxco, Guerrerro, Mexico

GALENA specimen gal-24
$ 50.00
$ 50.00
Dims: 2.7 x 2.3 x 1.4" (6.9 x 5.8 x 3.6 cm)
Wt: 9.47 oz. (268.6 g)
Naica, Chihuahua, Mexico
This specimen appears to consist mostly of Galena, though there is a substantial amount of sphalerite and some pyrite present. The Galena takes on a rather odd, highly warped form, as if many crystals "melted" together, but still retained a small amount of visible crystal form. Many of its edges are rounded and many faces are curved, but one can see that this curvature is due to "gradation" that consists of many tiny faces and edges. There is a small amount of fresh damage present in the form of straight cleavage faces and edges, but this is difficult to see. The Galena has the classic dark gray color and metallic luster of its kind, though its luster is brighter than many that I have seen. The sphalerite is similar in form, though somewhat better defined. One such sphalerite "crystal" seems to have thrust through the galena, which rings it (see the second image). The pyrite is scattered about the piece and takes on the form of tiny crystals. There is also a small amount of crystalline calcite and even a few small, colorless fluorites present. A wooden prop has been hot-glued to one side of the piece so that it will sit upright.


gal-24 ($ 50.00)
Naica, Chihuahua, Mexico

GALENA specimen gal-25
$ 26.00
$ 26.00
Dims: 2.6 x 1.9 x 1.4" (6.6 x 4.8 x 3.6 cm)
Wt: 8.44 oz. (239.4 g)
Pachapaque Mine, Ancash Department, Peru
Nearly all of the Galena on this small hand specimen is damaged and heavily cleaved into cubic fragments- only a small amount shows its natural cube-based and pseudohexagonal forms. It is difficult to discern individual crystals among the intact material due to intense intergrowth and dissemination of form. However, these crystals do possess well-defined edges and rather heavily patterned but clean faces that show the classic dark gray color and dull metallic luster. The cleaved areas show a brighter metallic luster and intense iridescence, with colors that range from deep red-violet to blue, green and even yellow. The intact crystals also are iridescent, but not nearly as much so as the cleaved spots. The Galena is accompanied by a few small crusts of tiny intergrown pyrite crystals and several small, milky quartz crystals that show good form. There is no actual host or base material present.
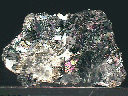
gal-25 ($ 26.00)
Pachapaque Mine, Ancash Department, Peru

GALENA specimen gal-26
$ 32.00
$ 32.00
Dims: 3.4 x 2.7 x 1.6" (8.6 x 6.9 x 4.1 cm)
Wt: 2 lbs., 11.7 oz. (1.239 kg)
Reynolds County, Missouri, U.S.A.
This hand specimen consists of a single large Galena crystal that is in reasonably good condition. It shows definite abrasions on a few edges and corners and appears to be cleaved on two faces- however, though these cleaved faces have a brighter metallic luster than the intact ones, the luster is partly dulled, making me believe that the breakage occurred prior to mining. The undamaged faces and edges show good cubic crystal form, with well-defined edges and clean faces that show heavy patterning. The crystal has the classic dark gray color and high density of its species and is accompanied by only a small amount of the original brown shale host rock.


gal-26 ($ 32.00)
Reynolds County, Missouri, U.S.A.

GALENA specimen gal-27
$ 32.00
$ 32.00
Dims: 3.2 x 2.6 x 2.4" (8.1 x 6.6 x 6.1 cm)
Wt: 12.1 oz. (343 g)
Pleasant Ridge, Indiana, U.S.A.
Likely the only Galena piece from Indiana in our stock, this hand specimen consists of several warped Galena cubes and cubo-octahedrons that rest on pyrite or marcasite crusts which partly coat the gray limestone base. The Galenas appear to be heavily weathered in some areas, but this may simply be intense intergrowth that makes them appear rounded and warped. They are in surprisingly good condition, however, and show very little damage. Several can be discerned as individuals and measure up to 0.3" (8 mm) along an edge. All have the classic dark gray color and slightly dull metallic luster that is standard for the species. They are accompanied by a substantial number of very small marcasite crystals and botryoidal crusts- these crusts are very dull, and may possibly be coated with siderite. The limestone base appears to be partly saturated with an oil or other liquid.

gal-27 ($ 32.00)
Pleasant Ridge, Indiana, U.S.A.

GALENA specimen gal-28
$ 28.00
$ 28.00
Dims: 4.1 x 2.6 x 1.5" (10.4 x 6.6 x 3.8 cm)
Wt: 7.5 oz. (212 g)
Elmwood Mine, Carthage, Smith County, Tennessee, U.S.A.
While there are only a few Galena crystals present on this hand specimen, I believe that they are not very common with respect to the other minerals found at this particular locality. The largest Galena formation is a badly damaged and incomplete cluster that rests at one end of the piece. The largest complete crystal measures 0.3 - 0.4" (8 - 10 mm) along an edge and is in excellent condition. All have the classic moderately dark gray color and dull metallic luster of their species and show excellent cubic crystal form, with well-defined edges and reasonably clean faces. They are accompanied by several warped and intergrown sphalerite crystals and several fluorites, almost all of which are broken. Some of the sphalerites appear to have grown over a few of the Galena crystals, enveloping them almost completely.


gal-28 ($ 28.00)
Elmwood Mine, Carthage, Smith County, Tennessee, U.S.A.

GALENA specimen gal-30
$ 40.00
$ 40.00
Dims: 2.3 x 2.3 x 1.5" (5.8 x 5.8 x 3.8 cm)
Wt: 5.8 oz. (164 g)
Pleasant Ridge, Indiana, U.S.A.
A seemingly weathered cluster of highly-intergrown Galenas rests on the brown limestone base of this piece. The Galena crystals are so heavily intergrown that it is difficult to isolate individual crystals. All have the classic gray coloration and slightly dull metallic luster. The limestone base contains a few crusts of tiny marcasite or pyrrhotite crystals and what appears to be crystalline and botryoidal siderite, though I cannot be sure.

gal-30 ($ 40.00)
Pleasant Ridge, Indiana, U.S.A.

GALENA specimen gal-31
$ 30.00
$ 30.00
Dims: 1.0 x 0.7 x 0.5" (2.5 x 1.8 x 1.3 cm)
Wt: 16.9 g w/ specimen box
Buick Mine, Reynolds County, Missouri
This thumbnail specimen is made up of several partly-intergrown, pseudo-hexagonal Galena blades. These blades are in good condition and show excellent form, including Spinel-Law twinning. All have the classic dark gray color and dull metallic luster of their species. The Galena is accopmanied by many tiny calcite rhombohedrons, and is glued into a plastic specimen box.

gal-31 ($ 30.00)
Buick Mine, Reynolds County, Missouri

GALENA specimen gal-32
$ 32.00
$ 32.00
Dims: 0.7 x 0.7 x 0.6" (1.8 x 1.8 x 1.5 cm)
Wt: 16.4 g w/ specimen box
Buick Mine, Reynolds County, Missouri, U.S.A.
A few pseudo-hexagonal tabular Galena crystals are intergrown to form this thumbnail specimen. These crystals do not exceed dimensions of 0.6 x 0.5 x 0.2" (1.6 x 1.3 x 0.4 cm) but are in excellent condition, showing little fresh damage, and have excellent form. The most prominent crystal is actually a Spinel-Law Twin, and there is evidence of twinning on other portions of the piece. They have the classic dark gray color and dull metallic luster of Galena and are dusted in spots with tiny bits of pyrite and sphalerite. The piece is hot-glued into a plastic specimen box.

gal-32 ($ 32.00)
Buick Mine, Reynolds County, Missouri, U.S.A.

$ 190.00
Dims: 3.5 x 2.7 x 1.1" (9.0 x 7.0 x 2.9 cm)
Wt: 5.7 oz. (161 g) w/ base
Madan Mining District, Rhodope Mountains, Plovdiv District, Bulgaria
This gorgeous cabinet specimen consists of a crystalline quartz crust on which rests a single parallel formation of intergrown Galena cubes - I suppose that this formation could be referred to as a single entity, however. It is in excellent condition, showing light damage on one side, and has an interesting and complex form that suggests formation under rather odd, complicated growing conditions - its edges are sharp, however, and its faces are exceptionally smooth, showing the standard dark silvery-gray color of the specie and an unusually bright metallic luster. It is accompanied by 3 chalcopyrite crystals, one of which is intergrown with the Galena and badly broken, and another of which is noticeably cracked. The two intact crystals have the standard deep golden-yellow color and metallic luster, and show excellent tetragonal disphenoidal form. A few small, heavily-intergrown sphalerites are also present on the quartz base, which is basically a druse of hundreds of small, well-formed crystals that are in good condition. The piece is hot-glued onto a square acrylic base.

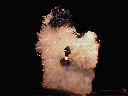
Madan Mining District, Rhodope Mountains, Plovdiv District, Bulgaria

GALENA specimen gal-34
$ 42.00
$ 42.00
Dims: 1.1 x 1.0 x 0.4" (2.7 x 2.5 x 0.9 cm)
Wt: 15 g
Buick Mine, Reynolds County, Missouri, U.S.A.
A couple of intergrown Galena crystals make up this piece. One of these crystals is much larger than the others and effectively makes up the entire piece, however. It is in good condition, showing some light damage in one area and has a good pseudohexagonal, tabular form that shows its Spinel-Law twinning. Its gray color and dull metallic luster are standard characteristics. The piece was hot-glued into a plastic specimen box, but came loose (the box is still included).
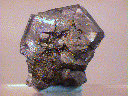
gal-34 ($ 42.00)
Buick Mine, Reynolds County, Missouri, U.S.A.

GALENA specimen gal-35
$ 200.00
$ 200.00
Dims: 5.8x3.0x2.5" (14.8x7.7x6.4 cm)
Wt: 1 lb 9.5 oz (721 g)
Osikovo, Bulgaria
This cabinet sized galena specimen is a little unusual, in that it is accompanied by dozens of radiated sprays of pale green colored quartz crystals. The galena is primarily in the form of intergrown and somewhat distorted cubes, with a few possible octahedrons. Most of the surfaces have a rather dull luster, although there are some surfaces with a metallic reflection. The color is the typical gray of natural (not cleaved) galena crystals.

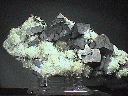
gal-35 ($200.00)
Osikovo, Bulgaria

GALENA specimen gal-36
$ 48.00
$ 48.00
Dims: 3.5x2.1x1.5" (9.0x5.4x3.9 cm)
Wt: 8.9 oz. (253g)
Mogila, Bulgaria
This hand specimen consists of several large rectangular galena crystals on a sphalerite matrix. There are also numerous calcite crystals in various forms present. The galena crystals have a dull metallic luster, and various shapes including cubes and rectangles. The largest crystal is rather odd - it is largely hollow, although it is also mostly buried under other crystals, so it cannot be examined in its entirety.

gal-36 ($ 48.00)
Mogila, Bulgaria

GALENA specimen gal-37
$ 75.00
$ 75.00
Dims: 3.8x1.8x0.9" (9.7x4.7x2.3 cm)
Wt: 3.5 oz. (99.8g)
Kruchevdol Mine, Madan, Bulgaria
This hand specimen has a nice presentation of galena crystals on a quartzite matrix. The galena crystals range up to 14mm in length, and have the standard silver-gray color and a decent metallic luster (not dull as is typical with natural galena crystal faces). The crystals are a combination cubic and octahedral - they look like cubes with pyramid terminations. The bottom of the specimen has hundreds of tiny crystals, which seem far too shiny to be galena (not counting cleavage faces). Their form is mostly cubic, and some faces are strongly striated. I am not sure what they are. Ther is also a small patch of something that looks like elemental silver.


gal-37 ($ 75.00)
Kruchevdol Mine, Madan, Bulgaria

GALENA specimen gal-38
$ 90.00
$ 90.00
Dims: 2.64x2.32x1.38" (6.7x5.9x3.5cm)
Wt: 5.71oz. (161.5g)
Huanzala Mine, Huallanca, Dos de Mayo, Huanuco, Peru
I really like this specimen, although several of its characteristics confuse me. The documentation from the miner says "Galena and Pyrite after Pyrrhotite", but I don't see the pyrrhotite crystal form. What I do see is blades (yes, flat thin blades like barite often forms) of tiny, pure pyrite crystals (these have a good, brassy color and decent sparkle). Intergrown with the pyrite are dozens of larger crystals of galena, although the shape of the crystals is neither cubic nor octahedral, rather they have a complex shape, probably a combination of cubes and octahedrons (some of the crystals almost look like metallic garnets). Further, the galena crystals have a high metallic luster, and most of them are irridescent blue (some more than others). Lastly, on top of the pyrite in many places is another mineral, which I have not identified - it looks black, and may be sphalerite.

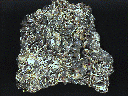
gal-38 ($ 90.00)
Huanzala Mine, Huallanca, Dos de Mayo, Huanuco, Peru

GALENA specimen gal-39
$ 240.00
$ 240.00
Dims: 5.59x2.49x1.79" (14.20x6.32x4.54cm)
Wt: 17.57oz (498g)
Madan Mining District, Rhodope Mountains, Plovdiv District, Bulgaria
This galena specimen presents its crystals atop a base of prismatic quartz crystals. The galena is relatively clean and lustrous, with the typical metallic gray color. No two galena crystals seem to have the same shape, although they are generically cubes with truncated points, such that (if any were complete) they would have 14 faces. But they are not complete, and some are more nearly cubes than others, and many show stepped growth patterns, a few to a large degree. Also, several of the galena crystals have significant cavities in one or more sides. The quartz crystals have milky bases and transparent tips. I like this specimen because it presents well.


gal-39 ($240.00)
Madan Mining District, Rhodope Mountains, Plovdiv District, Bulgaria

GALENA specimen gal-40
$ 150.00
$ 150.00
Dims: 2.81x2.00x1.57" (7.13x5.07x3.99cm)
Wt: 14.39oz (408g)
Kruchevdol Mine, Madan, Bulgaria
While there are no perfect cubes of galena, I really like this specimen. The galena may actually be dominated by a single crystal with an incredibly complex shape. From some angles all of the thousands of crystal facets have the same orientation, suggesting that they are parts of the same crystal. It is highly irregular, although composed of cubic sections. There is also several clusters of prismatic quartz crystals which have a frosted surface giving them a slightly milky appearance.


gal-40 ($150.00)
Kruchevdol Mine, Madan, Bulgaria

GALENA specimen gal-41
$ 110.00
$ 110.00
Dims: 3.76x2.16x1.71" (9.55x5.48x4.35cm)
Wt: 12.35oz (350g)
Madan, Bulgaria
This specimen displays nicely on its own, having two different relatively flat surfaces on which to stand. It has several centimeter sized cubes of galena, and dozens of crystals that are 4 to 6 mm in length. Accompanying the galena are scores of small prismatic quartz crystals, a matrix largely consisting of sphalerite, and many fine crusts of a white crystalline mineral that I have not identified.


gal-41 ($110.00)
Madan, Bulgaria

GALENA specimen gal-42
$ 72.00
$ 72.00
Dims: 4.60x1.99x1.48in (11.68x5.04x3.77cm)
Wt: 6.20oz (175.7g)
Madan, Bulgaria
This nice specimen has clusters of galena cubes perched on a quartz host rock. There are also some darker crystals of sphalerite present. While the bulk of the quartz is massive and colored green by chlorite, the top of the specimen has dozens pf pretty white prismatic quartz crystals that make a nice contrast to the metallic gray of the galena.


gal-42 ($ 72.00)
Madan, Bulgaria

GALENA specimen gal-46
$ 155.00
$ 155.00
dims mm=105.21x86.84x55.10
wt g=740
Petroviza Mine, Madan, Bulgaria
A fist sized piece of sphalerite is host to intergrown masses of pyrite and chalcopyrite, and on this lie a half-dozen large and very well formed galena crystals. There are a few tiny quartz crystals, and I would be surprised if other metallic sulfides could not be found with a detailed examination.


gal-46 ($155.00)
Petroviza Mine, Madan, Bulgaria

GALENA specimen gal-43
$ 30.00
$ 30.00
dims mm=94.73x78.36x43.31
wt g=605
Pine Point, North West Territories, Canada
Dozens of intergrown cubic crystals of galena cover most of one surface of this specimen. The galena has a finely pitted surface resulting in a dulled luster, except for a few shiney cleavage surfaces. The host rock is either calcite or dolomite which has mostly been etched away, also revealing hundreds of distorted translucent dirty-green crystals that look like olivine to me, but could conceivably be something else such as a green sphalerite.


gal-43 ($ 30.00)
Pine Point, North West Territories, Canada