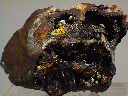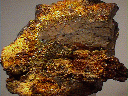The
Mineral CACOXENITE
The
Mineral CACOXENITE
- Chemistry: Fe24(AlO6)(PO4)17(OH)12 - 75H2O, Hydrated Iron Aluminum Phosphate Oxide Hydroxide.
- Class: Phosphates
- Uses: Only as mineral specimens.
Specimens
Cacoxenite is a mineral that is commonly an inclusion in quartz, especially amethyst. The inclusions in amethyst often detract from the amethyst as the brown acicular needles dampen the pure purple color to a less desirable brownish hue. The inclusions of cacoxenite will certainly ruin any chance for the host stone to become a gemstone. This is not to say that some specimens of cacoxenite included quartz have no value, for indeed some are actually enhanced with interesting surreal landscaping effects.
Cacoxenite on its own is appreciated as a scarce phosphate mineral and is known from classic phosphate localities. It is often associated with other attractive and rare phosphates and can therefore represent some very nice mineral specimens. These specimens can be quite popular and attractive with a silky luster and a typical yellow-brown color.
The rather daunting formula of cacoxenite, Fe24(AlO6)(PO4)17(OH)12 - 75H2O, can be represented in a more condensed and simpler version, Fe4(PO4)3(OH)3 - 12H2O. This shorter version is what is often used in some references. The shorter version is approximately one sixth of the larger one except for the addition of one aluminum and an adjustment to the number of oxygens and water molecules. This can be demonstrated:
- Fe24 (AlO6) (PO4)17 (OH)12 - 75H2O
- The aluminum group occupies a phosphate position in the structure and this can be shown as follows:
- Fe24 [(AlO6), (PO4)]18 (OH)12 - 75H2O
- Combine the 6 oxygens to 6 hydrogens from three water molecules to form 6 more OH's:
- Fe24 [Al, (PO4)]18 (OH)18 - 72H2O
- Then the formula can be shown as:
- Fe(6x4) [Al, (PO4)](6x3) (OH)(6x3) - 6x12H2O ;
- factoring out 6 and ignoring the aluminum gives:
- 6 x (Fe4 (PO4)3 (OH)3 - 12H2O)
This change requires the loss of three oxygens in order to balance the formula due to the change in charge from one negative 3 phosphate group to the negative 9 aluminum group. Natural cacoxenite contains one aluminum group for every 17 phosphates making the larger formula more accurate, if not less cumbersome.
PHYSICAL CHARACTERISTICS:
- Color is yellow-brown, brown, reddish-yellow, greenish-yellow or yellow.
- Luster is vitreous to silky.
- Transparency: Specimens are translucent to transparent.
- Crystal System is hexagonal.
- Crystal Habits include acicular radiating crystals often as inclusions, also massive, globular, botryoidal and fibrous tufts.
- Cleavage is poor.
- Fracture is fibrous.
- Hardness is 3 - 4
- Specific Gravity is approximately 2.3 (below average).
- Streak is yellow.
- Associated Minerals include quartz, hematite, strengite, beraunite, rockbridgeite and limonite.
- Notable Occurrences include Cornwall, England; Sweden; France; Hagendorf, Germany; Antwerp, New York; Pima County, Arizona; Indian Mountain, Alabama and Coon Creek Mine, Polk County, Arkansas, USA.
- Best Field Indicators are crystal habit, color, associations, density and streak.