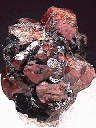
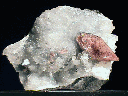
AXINITE |
THE TRICLINIC SYSTEM
|
| SOME TRICLINIC
MINERALS
|
The triclinic system is the lowest symmetry
system and contains only two symmetry classes.
One class contains only a center and the otherclass is
left with no symmetry what so ever. All crystallographic axes
are inclined with respect to each other, with no angles equaling 90 degrees.
Also all three axes are of differing lengths.
The symbology for these two classes is
bar 1, for the Pinacoid Class and just 1,
for the Pedion Class. The bar stands for the center,
a symmetrical inversion through the crystallographic
center of the crystal. Faces on one side of the crystal
are inverted on the other side. The inversion switches
everything about the individual faces; what is left is right
and what is up is down. The 1 refers to the one full rotation required
to repeat a face or edge about each crystallographic axis. In other words,
there is no rotational repeating of faces or edges.
The lack of symmetry gives rise to the thought that the triclinic system is the closest system to amorphism, having no form or order. It is actually the other way around. Triclinic minerals have a more sophisticated ordering of the atoms in their structure than other minerals. Ordering is a way of saying that the atoms are precisely placed into specific sites and these sites, in the case of triclinic minerals, are not symmetrically arranged. As an analogy think of an alphabetic filing system with lots of random, unsorted papers scattered among the files. A scattering of unsorted papers might produce a more or less even distribution of papers among the files and in turn form a more-or-less symmetrical distribution, at least to the size of each file. In other words, the A file has just as much chance to have say 10 papers in it as the B file or C file, etc. But if the papers are sorted alphabeticly, the distribution of papers is now irregular or asymmetrical, because files like A might contain a lot of papers and files like Q, hardly any. This kind of ordering occurs in mineral structures where various positions for atoms are assigned to specific elements. The more specific the site and the more specific the element that occupies those sites then the less symmetrical, but more ordered, the structure. The Pinacoidal Class
MICROCLINE
The Pedial Class
MURMANITE
|
|---|