 THE MINERAL ANHYDRITE or ANGELITE
THE MINERAL ANHYDRITE or ANGELITE
- Chemistry: CaSO4, Calcium Sulfate
- Class: Sulfates
- Uses: in the manufacture of some cement, a source of sulfate for sulfuric acid.
Specimens
Lilac blue Anhydrate is sometimes called Angelite, for it's "Angelic" color.
PHYSICAL CHARACTERISTICS:
- Color is ordinarily white, gray or colorless but also blue to violet.
- Luster is vitreous.
- Transparency crystals are transparent to translucent.
- Crystal System is orthorhombic; 2/m 2/m 2/m
- Crystal Habits include the tabular, rectangular box formed by three pinacoids, often elongated in one direction forming a prismatic crystal. Most commonly massive and granular.
- Cleavage is in three directions forming rectangles, but perfect in one, very good in another and only marginally good in the third direction.
- Fracture is conchoidal.
- Hardness is 3.5
- Specific Gravity is approximately 3.0 (average for translucent minerals)
- Streak is white.
- Associated Minerals are calcite, halite, and ocassionally sulfides such as galena and pyrite.
- Other Characteristics: some specimens fluoresce under UV light.
- Notable Occurances include Mexico; Peru; Germany and New Mexico.
- Best Field Indicators are crystal habit, rectangular and non-uniform cleavage and low density.
 Amethyst Galleries' Mineral Gallery MINERALS |

ANHYDRITE specimen anh-1
$ 37.50
$ 37.50
Dims: 3-5/8" x 2-1/4" x 1-3/8"
Wt: 5.4 oz
Naica, Mexico
This is one of a few specimens of excellent Anhydrite crystal clusters we have in stock. The crystals themselves possess the dimensions listed above, are a pale blue color, and have terminations that are coated with a crust of Calcite that is probably colored gray due to an inclusion of some form of sulfide.

anh-1 ($ 37.50)
Naica, Mexico

ANHYDRITE specimen anh-2
$ 45.00
$ 45.00
Dims: 4" x 2-3/4" x 1-1/4"
Wt: 9.9 oz
Naica, Mexico
The largest of our crystalline Anhydrite selection, this large cluster measures 4 inches long. The crystals are relatively well-exposed on one side of the pale-blue cluster, showing neatly squared-off terminations. The other side of the tabular cluster shows a crust of visible needle-like Calcite crystals, turned gray by an inclusion of a metallic sulfide.
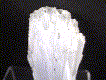
anh-2 ($ 45.00)
Naica, Mexico

ANHYDRITE specimen anh-3
$ 75.00
$ 75.00
Dims: 4-1/2" x 3-1/2" x 2-1/2"
Wt: 2 lbs., 8 oz
Naica, Chihuahua, Mexico
This specimen is certainly one of the most bizarre that I have seen. It has definitely had exposure to water during its formation! There is a cluster of Anhydrite crystals about 1-1/4 inch long and what looks like some very misshapen Calcite mixed with Pyrite and Rhodocrosite. These rest on a base of Galena that just barely shows some crystal form; it has been very worn and rounded. Under the Galena is a layer of Sphalerite, and Pyrite and Rhodocrosite follow in successive layers. This is a wonderfully weird piece of rock that is reminiscent of Peruvian material.

anh-3 ($ 75.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-4
$ 40.00
$ 40.00
Dims: 3.3" x 2.0" x 1.0"(8.4 x 5.1 x 2.5 cm)
Wt: 3.47 oz.(98.5 g)
Naica, Chihuahua, Mexico
Though showing some obvious cleaving at its tip, this Amhydrite crystal has otherwise excellent form. There are actually 4 or 5 crystals that make up this specimen: one large one, and 3 or 4 much smaller crystals that grow off of their larger relative. All of these smaller crystals show some heavy cleavage. All have a pale blue color, pearly luster, and a cloudy translucence. Small white calcite crystals grow on one side of each crystal. They are in the form of small scalenohedral "dogtooth" crystals, and have a slightly gray tinge to them. Other than these calcites, there is no host rock or other material to speak of.

anh-4 ($ 40.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-5
$ 22.00
$ 22.00
Dims: 2.3" x 1.8" x 1.0"(5.8 x 4.6 x 2.5 cm)
Wt: 2.44 oz.(69.4 g)
Naica, Chihuahua, Mexico
This specimen is composed of a cluster of cleaved but very clean Anhydrite crystals. Their form is quite good, and other than the cleavage, they are undamaged. They have an oblong orthorombic prismatic form with either stepped, angled terminations, or flat, shiny basal cleavage planes. Their color is a soft pastel blue, and they have a pearly luster and cloudy translucence. A few tiny crystalline bits of pyrite or a similar metallic sulfide are scattered on one side of the specimen, but there is no other material present.

anh-5 ($ 22.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-6
$ 33.00
$ 33.00
Dims: 3.0" x 2.3" x 1.0"(7.6 x 5.8 x 2.5 cm)
Wt: 3.53 oz.(100.1 g)
Naica, Chihuahua, Mexico
This specimen consists of two pale blue anhydrite crystals. One is much smaller than the other and has intergrown at an angle into its larger relative's base, nearly bisecting it. Thus, two portions of the crystal's termination are visible, one on each side of the larger crystal. Both crystals are in a flat, orthorombic tabular form and have sharp, complex terminations made up of many long, thin blades that grow out of their prisms. These prisms all have thin, flat shiny basal faces, showing this mineral's one perfect cleavage plane. Both crystals have a pearly luster and a cloudy translucence. Unlike most of the Anhydrite specimens that I have seen, this one is relatively pure; there is no coating of tiny calcite crystals or smattering of metallic sulfide on its faces or edges.


anh-6 ($ 33.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-7
$ 58.00
$ 58.00
Dims: 3.0" x 3.0" x 2.5" (7.6 x 7.6 x 6.4 cm)
Wt: 10.39 oz. (294.6 g)
Naica, Chihuahua, Mexico
This large piece consists of a large cluster of pale blue Anhydrite crystals that are coated with a crust of intergrown white calcite crystals. The Anhydrites show almost no damage, being protected by the calcite, and occur in an almost columnar form. They have a pearly luster on their prism faces and a vitreous luster on their basal faces, and are translucent. Patches of the calcite crust have a gray tinge, likely due to metallic sulfide inclusions. It is one of the larger Anhydrite specimens in our present stock.

anh-7 ($ 58.00)
Naica, Chihuahua, Mexico

$ 100.00
Dims: 4.6" x 3.9" x 3.4" (11.7 x 9.9 x 8.6 cm)
Wt: 2 lbs., 1.1 oz
Naica, Chihuahua, Mexico
One of the largest crystalline Anhydrite specimens in our present stock, this piece is made up of at least 3 separate, intersecting clusters of long, rectangular, orthorombic tabular crystals. The largest of these clusters is much larger than the other two, with measurements of 4.6 x 3.0 x 1.9" (11.7 x 7.6 x 4.8 cm). All have the standard pale gray-blue coloration, bright pearly luster, and cloudy, lined translucence that is common for Anhydrite crystals. Nearly half of each cluster's surface area is covered with a crust of small, white calcite crystals. Though the crystals are heavily intergrown, most still show a definite, steep, trigonal pyramidal form. They are colorless, somewhat clear, and also have a pearly luster. There is some noticeable damage to the specimen, but not much of it appears to be human-induced. It is quite a pretty piece.


Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-9
$ 50.00
$ 50.00
Dims: 7.2" x 6.6" x 0.8" (18.3 x 16.8 x 2.0 cm)
Wt: 2 lbs., 5 oz
Naica, Chihuahua, Mexico
This Anhydrite specimen consists of a section of a nodule of massive Anhydrite, which some people may call Angelite. It is in the form of a slice of a nodule, and accurately represents a cross-section. There is a thin layer of a tan-to-brown, dull host rock that made up the skin of the nodule, surrounding the interior, which consists of pure, massive Anhydrite. It shows no layering or patterning of any sort, and has a gray-blue coloration that somewhat resembles celestite's. The Anhydrite has a very cloudy visual texture to it that seems to affect the polished luster of both of the cut sides of the piece. It is definitely opaque at this thickness. There are round, felt pads attached to one polished face so that the piece can be used as a paperweight or table decoratio

anh-9 ($ 50.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-10
$ 45.00
$ 45.00
Dims: 5.5 x 2.2 x 0.8" (14.0 x 5.6 x 2.0 cm)
Wt: 6.01 oz. (170.3 g)
Naica, Chihuahua, Mexico
This large hand specimen consists of a spray of long, almost tabular Anhydrite crystals that radiate from a central point. These crystals are very long- some of them achieve lengths of up to 5.5" (14.0 cm)- and are in excellent condition, showing very little damage. All have excellent orthorhombic prismatic form- their edges are well-defined and their faces are moderately clean, possessing a rather dull pearly luster that appears almost silky in some respects. All have the standard pale blue coloration for pieces of this locality and an almost milky translucence. Many tiny, broken crystals are clustered together at the base of the spray, but there is no host rock present. Upon close examination of one side of the spray, however, one can see tiny smatterings of colorful chalcopyrite or bornite on a few of the blades.

anh-10 ($ 45.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-11
$ 45.00
$ 45.00
Dims: 4.6 x 2.3 x 1.0" (11.7 x 5.8 x 2.5 cm)
Wt: 5.52 oz. (156.5 g)
Naica, Chihuahua, Mexico
This hand specimen consists of a flat spray of thin Anhydrite blades. These blades reach lengths of up to 4.6" (11.7 cm) and are in very good condition, as some termination damage is noticeable. All have excellent orthorhombic form, with well-defined edges and clean faces that possess the almost silky luster that is characteristic of this species. They have the standard pale blue color and are quite translucent. A few small crystals extend in odd directions from parts of the spray, and a few areas are faintly dusted with almost microscopic chalcopyrite crystals. There is no host rock present, however.

anh-11 ($ 45.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-12
$ 48.00
$ 48.00
Dims: 5.4 x 2.3 x 1.1" (13.7 x 5.8 x 2.8 cm)
Wt: 9.55 oz. (271.0 g)
Naica, Chihuahua, Mexico
This hand specimen consists of at least 2 intersecting sprays of intergrown Anhydrite blades. The blades reach a maximum length of 5.4" (13.7 cm) and are so intergrown that it is difficult to determine their other dimensions. They are all in excellent condition, however, as they show almost no fresh damage. Their orthorhombic prismatic form is also excellent- even with their intergrowth, the crystals show very well-defined edges and clean faces that possess the nearly silky luster that is standard for this species. They also have the obligatory pale sky-blue coloration and cloudy translucence. Several very small calcite scalenohedrons are scattered on the Anhydrites- they are slightly rounded, but still show good form. There is no host rock present, of course.

anh-12 ($ 48.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-13
$ 30.00
$ 30.00
Dims: 3.5 x 2.2 x 1.0" (8.9 x 5.6 x 2.5 cm)
Wt: 1.97 oz. (55.8 g)
Naica, Chihuahua, Mexico
Two intersecting clusters of intergrown Anhydrite blades make up this specimen. The smaller of the two has dimensions of 2.1 x 0.9 x 0.4" (5.3 x 2.3 x 1.0 cm), whereas the larger measures 3.3 x 1.5 x 0.3" (8.4 x 3.8 x 0.8 cm). Their intersection is not very heavy, so neither mars the form of the other. Likewise, the intergrowth of each crystal does not really interfere with their orthorhombic prismatic forms, which are very good- their edges are well-defined and their faces are clean, possessing a pearly luster that looks nearly silky from some angles. They have the standard pale gray-blue coloration and are translucent. Both of the flat clusters are coated on one side by a druse of small, dirty calcite scalenohedra. There is no host rock present, however.

anh-13 ($ 30.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-14
$ 60.00
$ 60.00
Dims: 4.7 x 3.2 x 2.0" (11.9 x 8.1 x 5.1 cm)
Wt: 9.39 oz. (266.4 g)
Naica, Chihuahua, Mexico
This hand specimen consists of several radiating sprays of Anhydrite blades. These blades are generally in good condition, showing very little damage, and reach lengths of up to 4.8" (12.2 cm). Each cluster is made up of several intergrown Anhydrite blades that have excellent orthorhombic bladed form, with well-defined edges and clean faces that possess a pearly luster that appears nearly silky from some angles. All have the classic pale blue coloration with a hint of gray and are translucent. Tiny smatterings of colorful chalcopyrite are scattered on the crystals and a single cluster of small calcites is present, but there is no host rock.

anh-14 ($ 60.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-15
$ 60.00
$ 60.00
Dims: 5.3 x 3.4 x 3.2" (13.5 x 8.6 x 8.1 cm)
Wt: 1 lb., 3.2 oz. (545 g)
Naica, Chihuahua, Mexico
At least 8 discernable Anhydrite crystals are partly intergrown to form this large hand specimen. These crystals are generally in very good condition, though a few have flat, shiny basal faces that make me think they were cleaved (I am not certain about this, however). They reach lengths of up to 5" (12.7 cm) and have good orthorhombic prismatic form, though their faces and edges are not highly defined. All have the classic pale blue coloration and pearly luster of anhydrite, though a few faces appear to be a bit worn and dull. Each crystal is dimly to moderately translucent. There is no host rock of any sort present.

anh-15 ($ 60.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-16
$ 50.00
$ 50.00
Dims: 3.4 x 1.9 x 1.8" (8.6 x 4.8 x 4.6 cm)
Wt: 7.80 oz. (221.1 g)
Naica, Chihuahua, Mexico
This hand specimen consists primarily of 2 intersecting Anhydrite crystals that are similar in size, having dimensions of approximately 3.2 x 1.9 x 0.7" (8.1 x 4.8 x 1.8 cm). All are in good condition and have excellent orthorhombic prismatic form- their edges are well-defined and their faces are generally clean. They have the classic pale, sky-blue color and pearly luster of Anhydrite, and are translucent. They are accompanied by a few tiny pyrite crystals and several well-formed scalenohedral calcites, some of which are double-terminated. There is no host or base rock present, however.

anh-16 ($ 50.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-17
$ 25.00
$ 25.00
Dims: 3.5 x 1.8 x 1.1" (8.9 x 4.6 x 2.8 cm)
Wt: 2.50 oz. (71.0 g)
Naica, Chihuahua, Mexico
Several intergrown Anhydrite blades make up this small hand specimen. The largest of these blades has dimensions of 3.4 x 1.7 x 0.4" (8.6 x 4.3 x 1.0 cm), and like the others, is in very good condition, showing only a small amount of human-induced damage. All have excellent orthorhombic form, with well-defined edges and clean faces that possess the standard pearly luster. All have the classic pale blue color of Anhydrite and are translucent. Besides a few small, thin crusts of almost massive pyrite, there is no other material present.

anh-17 ($ 25.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-18
$ 30.00
$ 30.00
Dims: 3.5 x 1.7 x 1.6" (8.9 x 4.3 x 4.1 cm)
Wt: 2.01 oz. (57.0 g)
Naica, Chihuahua, Mexico
This small hand specimen consists of 4 partly intersecting Anhydrite crystals. Two of these crystals are quite small and are generally heavily intergrown with the largest crystal, which has dimensions of 3.0 x 1.1 x 0.5" (7.6 x 2.8 x 1.3 cm). All are in very good condition, showing only minor damage if any, and their orthorhombic bladed form is very good. They have the standard pale blue color and almost silky, pearly luster of their species, and are translucent. The larger crystals are both encrusted with small calcite scalenohedra on one side, but there is no base or host rock present.

anh-18 ($ 30.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-19
$ 32.00
$ 32.00
Dims: 2.8 x 1.9 x 1.2" (7.1 x 4.8 x 3.0 cm)
Wt: 6.80 oz. (192.0 g)
Naica, Chihuahua, Mexico
This specimen consists of a partly-cut, partly-polished chunk of massive Anhydrite, which is sometimes called Angelite. Obviously, the piece has no visible crystal form and is thus undamaged to all intents and purposes. Three flat faces have been cut into the piece, and one slightly convex face has been polished to a moderately high gloss. It has the classic pale blue color with a tinge of gray that is its hallmark, and is essentially opaque at this thickness. Several fracture lines are visible in the polished face, and there is a thin crust of a dull, pale brown material that coats about half of the uncut surface.

anh-19 ($ 32.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-20
$ 25.00
$ 25.00
Dims: 2.5 x 1.9 x 0.6" (6.3 x 4.9 x 1.5 cm)
Wt: 2.02 oz. (57.3 g)
Chihuahua, Mexico
This small hand specimen consists of a partly-polished chunk of massive Anhydrite, which is sometimes known as Angelite. Where rough, it appears to be coated with a thin layer of white, massive calcite. Cutting has revealed the pale, gray-blue Anhydrite, which is dimly translucent and has been polished to a moderate gloss. One small cut surface on the "underside" of the piece has not been polished

anh-20 ($ 25.00)
Chihuahua, Mexico

ANHYDRITE specimen anh-21
$ 36.00
$ 36.00
Dims:4.0x2.0x1.2" (10.1x5.1x3.0 cm)
Wt: 3.0oz. (85g)
Mina Singlo XX, Naica, Chihuahua, Mexico
This specimen consists of a delicate intergrown mass of anhydrite crystals. These crystals are a pale blue in color, and reach 3.0" (7.6cm) in length. Several crystals on this fragile specimen are broken, but in the confusion of intergrown crystals, it is relatively unnoticed.

anh-21 ($ 36.00)
Mina Singlo XX, Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-22
$ 60.00
$ 60.00
Dims: 5.0x2.75x1.8" (12.8x7.0x4.7 cm)
Wt: 10.6 oz. (300.0 g)
Naica, Chihuahua, Mexico
This is a very pleasing specimen of Angellite (a variety/trade name of anhydrite). The translucent pale-blue crystals show a radial habit, with curved basal cleavage planes. The back (natural, uncleaved) side of the specimen shows hundreds of intergrown crystals of anhydrite covered with a druze of what I believe to be calcite crystals.


anh-22 ($ 60.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-23
$ 60.00
$ 60.00
Dims:.7x2.4x2.1" (12.0x6.2x5.3 cm)
Wt: 11.7 oz. (331.8g)
Naica, Chihuahua, Mexico
This is a large hand specimen of Angellite, the pretty blue variety of anhydrite. The color is a pale blue, and the crystals are dimly translucent. There is an appealing play-of-light from the crystal faces. While the front of the specimen is dominated by 2 large crystals (and one smaller one), the back is a jumble of dozens or hundreds of anhydrite crystals, cemented together by countless small calcite crystals (which are colorless but look white in the aggregate).


anh-23 ($ 60.00)
Naica, Chihuahua, Mexico

ANHYDRITE specimen anh-25
$ 60.00
$ 60.00
Dims: 4.92x4.25x3.25" (12.50x10.78x8.25cm)
Wt: 19.29oz (547g)
Naica, Chihuahua, Mexico
This interesting cluster of anhydrite crystals has the characteristic light blue color and soft translucency of angellite. The crystals are strongly striated, and the groves and some surfaces are fille/coated with the original dirty-white host rock. The clean faces, for the most part, appear to be basal cleavage, except that they are all curved surfaces. Through these faces, one can see that the angellite is actually transparent but is nearly filled with minute milky inclusions.


anh-25 ($ 60.00)
Naica, Chihuahua, Mexico