|
To see our natural diamond specimens, see the
|
Diamond is the ultimate gemstone, having few weaknesses and many strengths. It is well known that Diamond is the hardest substance found in nature, but few people realize that Diamond is four times harder than the next hardest natural mineral, corundum (sapphire and ruby). But even as hard as it is, it is not impervious. Diamond has four directions of cleavage, meaning that if it receives a sharp blow in one of these directions it can cleave, or split. A skilled diamond setter and/or jeweler will prevent any of these directions from being in a position to be struck while mounted in a jewelry piece.
As a gemstone, Diamond's single flaw (perfect cleavage) is far outdistanced by the sum of its positive qualities. It has a broad color range, high refraction, high dispersion (fire), very low reactivity to chemicals, rarity, and of course, extreme hardness and durability. Diamond is the April Birthstone.
- Hardness: Diamond is a perfect "10", defining the top of the hardness scale, and by absolute measures four times harder than sapphire (which is #9 on that scale).
- Clarity: Diamond is transparent over a larger range of wavelengths (from the ultraviolet into the far infrared) than is any other solid or liquid substance - nothing else even comes close.
- Thermal Conductivity: Diamond conducts heat better than anything - five times better than the second best element, Silver!
- Melting Point: Diamond has the highest melting point (3820 degrees Kelvin)
- Lattice Density: The atoms of Diamond are packed closer together than are the atoms of any other substance
- Tensile Strength: Diamond has the highest tensile strength of any material, at 2.8 gigapascals. However, that does not quite translate into the strongest rope or cable, as diamond has cleavage planes which support crack propagation. The strongest ropes can likely be made from another carbon material, carbon nanotubes, as they should not suffer from the effects of cracks and break. Still, if a long, thin, perfect crystal of diamond could be manufactured, it would offer the highest possible pulling strength (in a straight line - don't try to tie it in a knot!)
- Compressive Strength: Diamond was once thought to be the material most resistant to compression (the least compressible). It is the material that scientists use to create the greatest pressures when testing matter. However, the rare metal Osmium has recently been shown to be even less compressible (although it is not as hard as diamond). Diamond has a bulk modulus (reciprocal of compressibility) of 443 GigaPascals (GPa). The bulk modulus of the metal osmium has recently been found to be 476 GPa, about 7% greater than diamond.
Diamond is a polymorph of the element carbon. Graphite is another polymorph. The two share the same chemistry, carbon, but have very different structures and properties.
- Diamond is hard, Graphite is soft (the "lead" of a pencil).
- Diamond is an excellent electrical insulator, Graphite is a good conductor of electricity.
- Diamond is the ultimate abrasive, Graphite is a very good lubricant.
- Diamond is transparent, Graphite is opaque.
- Diamond crystallizes in the Isometric system and graphite crystallizes in the hexagonal system.
- Somewhat of a surprise is that at surface temperatures and pressures, Graphite is the stable form of carbon. In fact, all diamonds at or near the surface of the Earth are currently undergoing a transformation into Graphite. This reaction, fortunately, is extremely slow.
Other polymorphs of carbon include:
- carbon nanotubes
- buckyballs
- graphene
Diamonds are found in kimberlite pipes (the cores of certain volcanoes originating beneath thick plates of the Earth's crust), and in alluvial deposits resulting from the erosion of those pipes. Nanodiamonds are also found as presolar grains in meteorites, and presumably in asteroids and comets.
For natural diamond mineral specimens, see our
PHYSICAL CHARACTERISTICS:
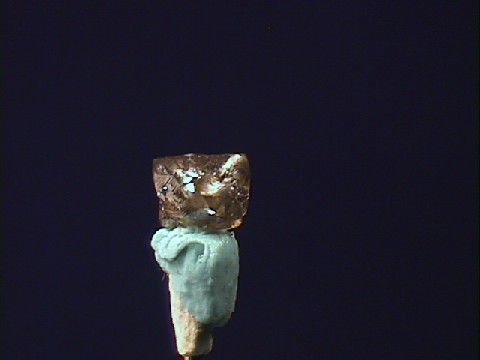
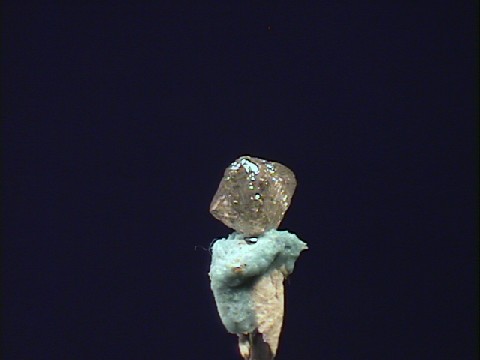
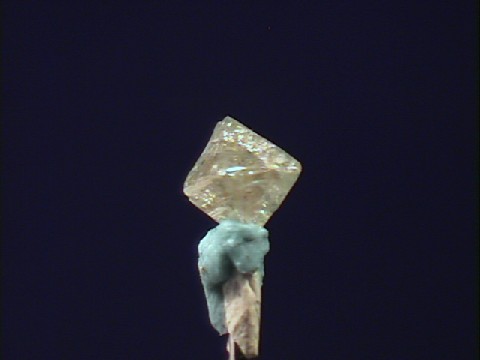
- Color is variable and tends toward pale yellows, browns, grays, and also white, blue, black, reddish, greenish and colorless.
- Luster is adamantine to waxy.
- Transparency crystals are transparent to translucent in rough crystals.
- Crystal System is isometric; 4/m bar 3 2/m
- Crystal Habits include isometric forms such as cubes and octahedrons, twinning is also seen.
- Hardness is 10
- Specific Gravity is 3.5 (above average)
- Cleavage is perfect in 4 directions forming octahedrons.
- Fracture is conchoidal.
- Streak is white.
- Associated Minerals are limited to those found in kimberlite rock, an ultramafic igneous rock composed mostly of olivine.
- Other Characteristics: refractive index is 2.4 ( very high), dispersion is 0.044, fluorescent.
- Notable Occurrences include South Africa and other localities throughout Africa, India, Brazil, Russia, Australia, and Arkansas.
- Best Field Indicator is extreme hardness.
 THE MINERAL DIAMOND
THE MINERAL DIAMOND