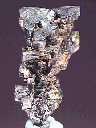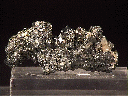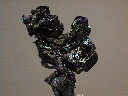The Mineral ACANTHITE/ARGENTITE
The Mineral ACANTHITE/ARGENTITE
- Chemistry: Ag2S, Silver Sulfide
- Class: Sulfides
- Uses: An ore of silver and as mineral specimens.
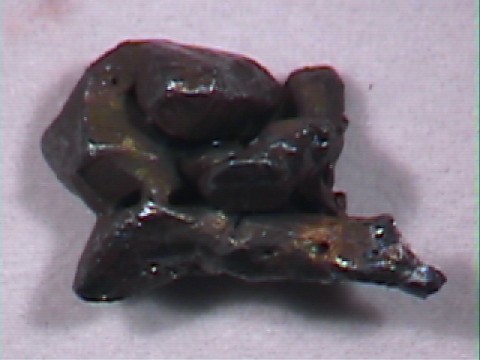
Specimens
Acanthite is often confused with the name argentite and it is no wonder. Several mineral guides interchange the names or combine the names as is done here. But the proper mineral name when referring to Ag2S at room temperatures is acanthite. Argentite is a name applied to one polymorph (meaning many shapes) of Ag2S. Acanthite and argentite have the same chemistry , Ag2S, but different structures. Argentite has an isometric structure and is only stable at temperatures above 173 degrees Celsius and if cooling from a melt, will form isometric crystals such as cubes, octahedrons and dodecahedrons. Upon cooling to below 173 degrees Celsius, argentite transforms from its isometric structure to the monoclinic structure of acanthite. The transformation often distorts the crystals to unrecognizable shapes, but some will still have an overall isometric crystal shape. These crystals are called pseudomorphs (false shapes) as they are actually acanthite's crystals in the shape of argentite's crystals. Argentite has been historically used when referring to these crystals, but the acknowledgment of the true identity of the mineral requires the naming of these crystals (at lower temperatures) as acanthite.
Acanthite, in addition to the crystals discussed above, forms interesting groupings of crystals. The crystals themselves are often distorted, but they group together into some intricate arborescent (branching) structures.
Argentite comes from the Latin word for silver, argentum, from which the chemical symbol for silver, Ag, is also derived. Acanthite comes from the Greek word for thorn, acantha, in allusion to its typical crystal habits.
Acanthite is generally easily identified although it may appear like galena and other silver sulfide minerals. The crystal habits discussed above are usually diagnostic enough, however the sectility test may be needed in some cases. Acanthite is sectile which means that it can be cut into by a knife just like lead. Acanthite is generally a very valuable mineral specimen, due mostly to the high silver content and the rarity of good crystals. It is a pleasure to own fine acanthite specimens once they are finally procured.
PHYSICAL CHARACTERISTICS:
- Color is lead gray to black.
- Luster is metallic.
- Transparency: Crystals are opaque.
- Crystal System: Monoclinic; 2/m below 173 degrees Celsius (acanthite) and isometric; 4/m bar 3 2/m above (argentite).
- Crystal Habits include rarely well formed pseudo: cubes, octahedrons and dodecahedrons. Non-argentite crystals (those that formed below 173 degrees Celsius) of acanthite tend to be of a slender prismatic habit. More commonly found massive and as coatings or as arborescent (branching) and reticulated groups.
- Cleavage is absent.
- Fracture is conchoidal.
- Hardness is 2.5 - 3
- Specific Gravity is approximately 5.5 - 5.8 (slightly heavy even for metallic minerals).
- Streak is a shiny black.
- Other Characteristics: Sectile, meaning it can be cut with a knife like lead and fresh shiny surfaces will eventually form a dull coating after prolonged exposure to light (can be removed by ultrasonic treatment).
- Associated Minerals include silver, quartz, bornite, gold, galena, proustite, pyrargyrite, stephanite and other silver sulfide minerals.
- Notable Occurrences include Guanajuato, Mexico; Freiberg and Saxony, Germany; Cobalt, Ontario, Canada; Comstock Lode, Nevada and Butte, Montana, USA; Cornwall, England; Chile; Peru; Bolivia and especially Kongsberg, Norway.
- Best Field Indicators are crystal habit, density, softness, sectility, association with other silver sulfosalts and color.