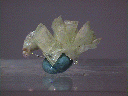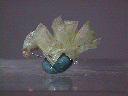 The
Mineral PARAVAUXITE
The
Mineral PARAVAUXITE
- Chemistry: FeAl2(PO4)2(OH)2 - 8H2O, Hydrated Iron Aluminum Phosphate Hydroxide.
- Class: Phosphates
- Group: Paravauxite.
- Uses: Only as mineral specimens.
Specimens
Paravauxite is closely related to its basic namesake vauxite. Paravauxite appears to be different from vauxite only in the number of water molecules in the structure. Paravauxite has eight water molecules while vauxite has only six in its formula. It is the presence of these water molecules that alters the structure to the point that paravauxite has perfect cleavage while in sharp contrast, vauxite has no cleavage at all. Cleavage is one property that is undeniably tied to the structure of a mineral. The only other strikingly different property is paravauxite's green to colorless color verses vauxite's blue color.
Paravauxite is the name of a somewhat rare phosphate mineral and an obscure mineral group. The Paravauxite Group is composed of other triclinic phosphates that have the following general formula:
XY2(PO4)2(OH)2 - 8H2O
or XY2(PO4)2(OH)3 - 7H2O
The X ion can be either iron(+2), magnesium, manganese or iron(+3) while the Y ion can be either aluminum, iron(+3) or to a lesser extent, chromium. The second formula is used when the X ion is the iron(+3) in stead of the iron(+2) and the additional positive charge requires the additional hydroxide and one less water molecule.
These are the members of the Paravauxite Group with their respective formula:
Gordonite MgAl2(PO4)2(OH)2 - 8H2O- Laueite MnAl2(PO4)2(OH)2 - 8H2O
- Paravauxite FeAl2(PO4)2(OH)2 - 8H2O
Sigloite FeAl2(PO4)2(OH)3 - 7H2OUshkovite MgFe2(PO4)2(OH)2 - 8H2O
PHYSICAL CHARACTERISTICS:
- Color is colorless, white or pale green.
- Luster is vitreous to pearly.
- Transparency: Specimens are translucent to transparent.
- Crystal System is triclinic.
- Crystal Habits include tabular crystals and radiating fibrous masses.
- Cleavage is perfect.
- Fracture is conchoidal.
- Hardness is 3
- Specific Gravity is approximately 2.4 (average).
- Streak is white.
- Associated Minerals include cassiterite, metavauxite, vauxite, wavellite, quartz and limonite.
- Notable Occurrences are limited to the famous tin deposits at Llallagua, Potosi, Bolivia and a few minor localities around the world.
- Best Field Indicators are color, locality, crystal habit, associations and perfect cleavage.