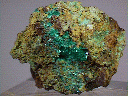THE
MINERAL CHALCOPHYLLITE
THE
MINERAL CHALCOPHYLLITE
- Chemistry: Cu18Al2 (AsO4)3(SO4)3(OH)27 - 33H2O , Hydrated Copper Aluminum Arsenate Sulfate Hydroxide
- Class: Phosphates
- Subclass: Arsenates
- Uses: a very minor ore of copper and as mineral specimens.
Specimens
Chalcophyllite, which is named from the Greek words for copper and leaf, is a rare secondary mineral that forms in the oxidation zone of copper ore deposits. It is a great collection mineral because it has a high luster, an attractive blue-green to green color and it forms nice tabular six-sided crystals that can be arranged into rosettes. Its crystals are similar to those of spangolite. Chalcophyllite has a green streak and that combined with its crystal habit make chalcophyllite difficult to misidentify.
Chalcophyllite is yet another in the long list of rare and classic
Chalcophyllite is unusual in that it has three different anion groups in its chemistry. Most minerals have just one principle anion group with possibly some hydroxides or halides along for the ride. Classifying those minerals is relatively easy as they are generally classed by their most complex or highest electronegative anion group. In the case of chalcophyllite, it has the same number of arsenate anion groups as sulfate anion groups. Should it be classified in the Sulfate Class or as an arsenate? Mineralogists prefer to classify it as a phosphate (where arsenates are placed) because the arsenate group has a higher negative charge (-3) than the sulfate group (-2).
Another unusual aspect of chalcophyllite's chemistry is its large amount of hydroxides and water molecules. Half of this mineral is either water or hydroxide! Generally copper minerals have a higher than average density, but with a specific gravity of 2.4 - 2.7, chalcophyllite is actually below average.
PHYSICAL CHARACTERISTICS:
- Color is blue-green to emerald green.
- Luster is adamantine, vitreous to pearly.
- Transparency crystals are transparent to translucent.
- Crystal System is trigonal; bar 3.
- Crystal Habits include tabular to platy crystals and lamellar masses. Some specimens have been partially pseudomorphed by chrysocolla and appear much duller in luster.
- Cleavage: is perfect in one direction (basal).
- Fracture: Uneven.
- Hardness is 2
- Specific Gravity is approximately 2.4 - 2.7 (light for copper minerals).
- Streak is green.
- Associated Minerals include
cornubite ,tyrolite , cuprite, caledonite, malachite, chalcopyrite, chrysocolla,devilline andconnellite . - Notable Occurrences are limited to Chile; France; Germany; Wheal Gorland, Wheal Unity and other mines in
Cornwall , England and Arizona, Utah and Nevada, USA. - Best Field Indicators are crystal habit, streak, locality, associations, density and color.