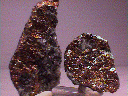THE
MINERAL NICKELINE
THE
MINERAL NICKELINE
- Chemistry: NiAs, Nickel Arsenide.
- Class: Sulfides
- Subclass: Arsenides
- Group: Nickeline
- Uses: As a minor ore of nickel and as mineral specimens.
Specimens
Nickeline has somewhat of an identity crisis. It is alternatively known as niccolite (mostly in Europe). Reference books often use Nickeline (Niccolite) or Niccolite (Nickeline) as a heading. The growing trend and "official" name is nickeline as is used here.
Its first name was the German term "Kupfer nickel" or copper nickel. It sounds like some sort of alloy. It actually was a term of derision because nickel meant "nixes" or underground goblins. The miners in Germany who first saw the copper-red metallic mineral were convinced the mineral was a rich ore of copper. Instead, try as they might, no copper was to be produced from the nickel arsenide. The metal that was produced from the mineral was found to be useful and valuable and it is from this unflattering term that the metal received its name, nickel (Ni).
Nickeline is also the name of a group of hexagonal minerals of which the mineral nickeline is one of the more common members. Members of the Nickeline Group have a very simple formula, namely: AX. Where the metal ion, A, is either cobalt, palladium, platinum, nickel and/or iron and the X can be either arsenic, selenium, bismuth, tellurium and/or antimony with some members having some sulfur. All members of the group have nickeline's basic structure. They are all hexagonal and except for nickeline and pyrrhotite, are very uncommon to rare in occurrence.
These are the members of the Nickeline Group:
Breithauptite (Nickel Antimonide)Freboldite (Cobalt Selenide)Imgreite (Nickel Telluride)Langistite (Cobalt Nickel Arsenide)- Nickeline (Nickel Arsenide)
- Pyrrhotite (Iron Sulfide)
Sederholmite (Nickel Selenide)Stumpflite (Platinum Antimonide Bismuthinide)Sudburyite (Palladium Nickel Antimonide)
Pyrrhotite has hexagonal and monoclinic components. The hexagonal components are placed in the Nickeline Group, but most natural pyrrhotite is a mixture of both components.
Nickeline's structure is fairly simple with the nickel ions, in hexagonal packing, forming stacked identical layers parallel to the major six-fold axis. The arsenic ions rest between the nickel ion layers, but in a staggered or alternating sequence such that the next layer is not in the same position as the previous layer. Each metal ion is surrounded by six arsenics; three below and three above. The major axis is actually a six-fold screw axis (helix) about the nickel ions.
A screw axis is an axis that has a rotation and a translation in the major axis direction. So, in this case, the axis will start with an arsenic ion at the base level. A rotation about the axis of 60 degrees and then movement (approximately 2.5 angstroms) along the axis produces the next arsenic ion. If this is done a total of six times, then the entire structure and consequently the symmetry of nickeline is reproduced. All members of the Nickeline Group share this basic structure, but with their own combination of elements of course.
Nickeline is not exactly a common mineral, but is found with other nickel and cobalt sulfide ores and thus it is included with them when mined for their various ores. This makes nickeline a minor ore of nickel and commercially important is some localities. The sulfides are usually hydrothermal in origin, although nickeline is also found in basic igneous rocks such as gabbros. Although crystals of nickeline are very scarce, some formations of nickeline can be attractive and an interesting addition to someone's collection.
PHYSICAL CHARACTERISTICS:
- Color is copper-red to pink.
- Luster is metallic.
- Transparency: Crystals are opaque.
- Crystal System: Hexagonal; 6/m 2/m 2/m
- Crystal Habits are limited to mostly massive and granular components of massive hydrothermal sulfide rocks and gabbros, but a few localities have produced good pyramidal or tabular crystals. Columnar and reniform habits are also seen. Rare fourling twins are also seen.
- Cleavage: Not observed.
- Fracture: Uneven.
- Hardness is 5 - 5.5
- Specific Gravity is approximately 7.8 (well above average for metallic minerals)
- Streak: Dark brown to black.
- Other Characteristics: Will often develop a dark tarnish on fresh surfaces and weathered surfaces may have a coating of the green nickel arsenate, annabergite. Upon heating a garlic odor maybe observed due to the arsenic content. Crystals tend to be striated.
- Associated Minerals include arsenopyrite,
barite,
silver,
annabergite,
cobaltite,
pyrrhotite,
pentlandite,
chalcopyrite,
breithauptite andmaucherite . - Notable Occurrences include the Natsume nickel mine, Japan and at Mansfeld and Eisleben, Germany; Franklin, New Jersey; California and Colorado, USA; Styria, Austria; Sinaloa, Mexico; Talmessi, Anarak, Iran and at Great Slave Lake, Northwest Territories, Canada; Cobalt and Sudbury, Ontario, Canada as well as some sites in England, France, Morocco, Russia and southern Australia.
- Best Field Indicators are color, density, associations, streak, hardness and odor when heated.