THE MINERAL REALGAR
THE MINERAL REALGAR
- Chemistry: AsS, Arsenic Sulfide
- Class: Sulfides and Sulfosalts
- Uses: A major ore of arsenic, formerly used for pigments, firework coloring agent and as mineral specimens.
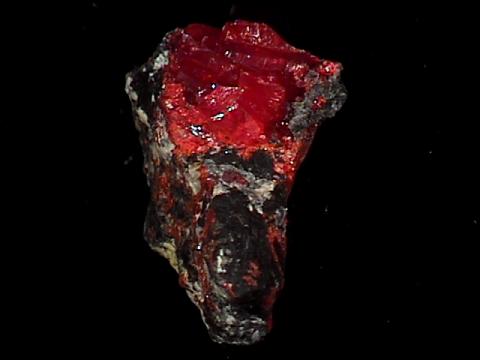
Specimens
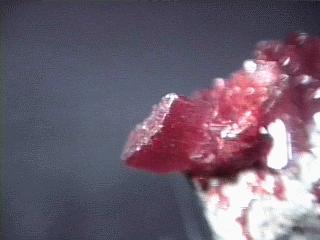
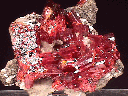
Realgar occurs in hydrothermal veins with valuable metal sulfide ores and its bright red color can be an aid to prospectors. It also can be found in hot spring deposits and as a volcanic sublimate product (crystallizing from vapors). Realgar gets its name from the Arabic words for "powder of the mine" (rahj al ghar). Realgar is famous for some wonderfully beautiful specimens. Some specimens can have a deep ruby red color with an amazing clarity and a high luster. The color of realgar is truly something to appreciate and cherish. But realgar's beauty is sometimes fleeting.
It is an unstable mineral and will alter to a different mineral,
PHYSICAL CHARACTERISTICS:
- Color is orange to red.
- Luster is resinous, adamantine to sub-metallic.
- Transparency: Crystals are translucent to transparent.
- Crystal System: Monoclinic; 2/m.
- Crystal Habits: include prismatic striated crystals with a rounded diamond-like cross-section. They are terminated by a wedge-like dome. Also found as grains, crusts and earthy masses.
- Cleavage is good in one direction.
- Fracture is subconchoidal.
- Hardness is 1.5 - 2
- Specific Gravity is 3.5 - 3.6
- Streak is orange to orange-yellow.
- Other Characteristics: Realgar is unstable in light; specimens should be stored in complete darkness, rarely some specimens fluoresce under UV light and crystals are pleochroic between dark red and orange red.
- Associated Minerals almost always include orpiment, also calcite, stibnite and other metal sulfide ores.
- Notable Occurrences include most importantly Hunan Province, China; but also Switzerland; Japan; Macedonia; Mercur, Utah, USA; Romania and many other localities.
- Best Field Indicators are of course color as well as crystal habit, association with orpiment, softness and luster.