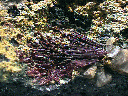 THE
MINERAL KERMESITE
THE
MINERAL KERMESITE
- Chemistry: Sb2OS2, Antimony Oxysulfide.
- Class: Sulfides
- Uses: A very minor ore of antimony and as mineral specimens.
Specimens
Kermesite is an unusual sulfide mineral. A close look at the formula can confirm this. There is an oxygen nested between two antimonies and two sulfurs. Kermesite, it turns out, is an intermediate oxidation product between stibnite whose formula is Sb2S3, and various antimony oxides such as senarmontite, Sb2O3; valentinite, also Sb2O3; and stibiconite, Sb3O6(OH). Stibnite when exposed to oxygenated fluids under the right conditions will slowly transform into kermesite as the an oxygen replaces one of the sulfurs in the formula. Usually the oxidation is completed to the point that the sulfurs are completely replaced by oxygen, but in some rare cases the oxidation stops short and forms kermesite. This is fortunate for mineral collectors!
Kermesite is a truly colorful mineral. Its bright red color has been described as a cherry red and that is pretty accurate. The color is caused by the stibnite and is therefore inherent in the mineral. Alternate names or nick-names for kermesite have been used such as red antimony, purple blende and a non-colorful nick-name antimony blende.
PHYSICAL CHARACTERISTICS
- Color is cherry red to red.
- Luster is adamantine.
- Transparency crystals are translucent to rarely transparent.
- Crystal System is triclinic, bar 1 (pseudo-monoclinic).
- Crystal Habits include sprays or tufts of aggregated prismatic crystals and as crusts.
- Cleavage is perfect in one direction.
- Hardness is 1.0 - 1.5
- Specific Gravity is approximately 4.5 - 4.8+ (heavy for translucent minerals)
- Streak is brownish red.
- Associated Minerals include stibnite and various antimony oxides such as senarmontite, valentinite and stibiconite.
- Notable Occurrences include Wolfe County, Quebec, Canada; Nova Scotia; Algeria and Sonora, Mexico.
- Best Field Indicators are color, luster, associations, softness and streak.






















