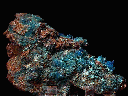THE
MINERAL CHALCANTHITE
THE
MINERAL CHALCANTHITE
- Chemistry: CuSO4 - 5H2O, Hydrated Copper Sulfate.
- Class: Sulfates
- Group: Chalcanthite
- Uses: A minor ore of copper, various chemical uses and as mineral specimens.
Specimens
Chalcanthite is one of only a few water soluble sulfate minerals. This fact drives much of what is interesting about this mineral. It forms in the near-surface secondary oxidation zone of copper deposits usually late in the development of these deposits. Since it is so soluble, it may crystallize, dissolve and recrystallize again and again before the deposit is discovered.
In wetter regions, chalcanthite is not found in large amounts (originally), but in arid regions, such as in Chile, chalcanthite is a major ore. Any sulfate rich ground water that might leach out copper from other copper minerals, will crystallize chalcanthite when the water has a chance to evaporate. In many copper mines, chalcanthite is an ongoing precipitate forming blue encrustations, crystal aggregates and stalactites right on the sides of the mine's shafts.
It is this ease of crystallization that is the bane of natural chalcanthite crystals, at least with respect to mineral collectors. More often than not, excellent crystals for sale from mineral dealers are fakes or more specifically, artificially grown crystals from a solution of copper sulfate in someone's house or shop. If they are natural, they often have such a wonderful color, striking form and beautiful clarity, that they are then deemed too perfect to be real and thus regarded as fakes when they actually are not. What's a dealer to do?In any respect, chalcanthite's solubility is its other enemy. Many fine specimens in museums and in private collections have met their doom as they slowly absorb water from the air and then lose it again. Over time, this cycle leads to the destruction of what once might have been a world class specimen into a pile of bluish dust. Curators and concerned collectors store their specimens in sealed containers with desiccants to postpone these destructive effects. Some chalcanthite seems resistant to water absorption and this resistance seems tied to the purity of the specimen, especially the lack of iron impurities.
Chalcanthite does have useful purposes or at least solutions of copper sulfate do. It is a wonderful tool to use to teach children how some crystals form. In a class room or at home with adult supervision, chalcanthite crystals can form easily over a few days time from a warm solution of copper sulfate left to evaporate. The always attractive blue color and the sparkling crystals help to keep the children interested. Adult supervision is strongly suggested as chalcanthite and copper sulfate solutions are poisonous.
There are many chemical uses for copper sulfate solutions. Copper sulfate solutions and crystals are a staple in well stocked chemistry labs. Metallic copper can be obtained from copper sulfate solutions by adding metallic iron, a process used in the mining and processing of chalcanthite. As a poison, copper sulfate solutions or crystals were used to clear ponds and waterways of plant growth, but this practice has stopped due to environmental concerns.
Identification of the mineral chalcanthite is generally pretty easy. Its bright blue color can be dulled on natural specimens, but it is otherwise very distinctive. Its solubility is also key if this test can be done with a small unnecessary fragment of the specimen in question. The resulting solution should turn blue. Another relatively common soluble sulfate is melanterite, FeSO4 - 7H2O, but it is generally greener.
Taste is a test that is used for some minerals such as halite and can be used on chalcanthite. Chalcanthite has a sweet metallic taste that is distinctive. However, it is not recommended as a test to be done casually for as was stated, chalcanthite is poisonous! If it is necessary, use a tip-of-the-tongue technique to minimize the risk.
Chalcanthite loosely translated from the Greek means copper flower. An apt name for this attractive mineral. Synonyms include "blue stone" and "copper vitriol". Chalcanthite is the name of a group of only four triclinic sulfates of which chalcanthite is its only common member. Other members have in place of copper ions of iron, manganese and magnesium.These are the members of the Chalcanthite Group:
- Chalcanthite (Hydrated Copper Sulfate)
Jokokuite (Hydrated Manganese Sulfate)Pentahydrite (Hydrated Magnesium Sulfate)Siderotil (Hydrated Iron Sulfate)
Specimens of chalcanthite are one-of-a-kind and are generally affordable. Natural specimens from an honest dealer are preferred, but are hard to get. Fakes (non-natural crystals), when sold as such, have their brilliant color and attractiveness to at least deserve consideration for placement in someone's collection.
PHYSICAL CHARACTERISTICS:
- Color is a bright and deep blue.
- Luster is vitreous.
- Transparency: Crystals are transparent to translucent.
- Crystal System is triclinic; bar 1.
- Crystal Habits include rare, individual, natural crystals showing well formed slanted prismatic, tabular or lense-shaped forms, more commonly aggregated into columnar, curved, parallel growth structures. Also found as encrusting, stalactitic and granular masses and as vein filling deposits.
- Cleavage is poor (basal).
- Fracture is conchoidal.
- Hardness is 2.5
- Specific Gravity is approximately 2.2 - 2.3 (noticeably below average).
- Streak is pale blue to colorless.
- Other Characteristics: Is very soluble in water. A fact that is a detriment to most collection specimens as they may absorb water from the air and deteriorate over time. The taste has a sweet metallic character, but do not do this test often or with more than just a tip-of-the-tongue technique and only if needed to confirm an identification as chalcanthite is poisonous!
- Associated Minerals are brochantite, calcite , melanterite, aragonite, malachite and chalcopyrite.
- Notable Occurrences include Chuquicamata and El Teniente (Sewell), Chile; Minas de Rio Tinto, Spain; England; Germany and Ireland and in the United States, Bigham Canyon, Utah; Ducktown, Tennessee; Imlay, Pershing County, Nevada; Arkansas; California; New Mexico and Arizona.
- Best Field Indicators are crystal habit, low density, associations, solubility in water, taste and color.