THE MINERAL SAL
AMMONIAC
THE MINERAL SAL
AMMONIAC
- Chemistry: NH4Cl, Ammonium Chloride.
- Class: Halides
- Uses: As mineral specimens.
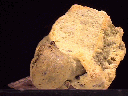
Specimens
Sal ammoniac is certainly an oddball mineral. It is composed of ammonium, NH4, and this alone is odd enough. Sal ammoniac is one of the most common and most well known of the ammonium-bearing minerals. These are some other ammonium bearing-minerals:
Boussingaultite (Hydrated Ammonium Magnesium Sulfate)Cryptohalite (Ammonium Silicon Fluoride)Guanine (Carbohydrate Ammonium Nitrogen Oxide)Struvite (Hydrated Ammonium Magnesium Phosphate)Tschermigite (Hydrated Ammonium Aluminum Sulfate)
Sal ammoniac forms on volcanic rocks near fume releasing vents. There is no liquid phase as the mineral crystallizes from these fumes in a process called sublimation. The crystallization occurs as the gases are escaping and crystals tend to be short-lived. Sal ammoniac is very soluble in water and crystals will be removed during the first rain of their existence, so to speak, if they are not removed by collectors first.
Other possible natural occurrences exist from underground burning coal seams. Alexander the Great is said to have found sal ammoniac crystals in a cave in a region that is now Tadzhikistan. The region was plagued by underground burning coal seams.
Sal ammoniac can be produced artificially and has its uses. When ammonia fumes are blown across hydrochloric acid; sal ammoniac fumes are produced. The technique is sometimes used to produce sal ammoniac coatings on dark objects that are about to be photographed. This will often enhance a difficult to photograph object by adding detail to the subject.
Natural crystals of sal ammoniac have an unreal or unnatural character to them. They are so small, delicate, intricate and at times quite beautiful that they just do not seem to be like other minerals. But it is sal ammoniac's natural methods of origin that lend themselves to produce these one-of-a-kind specimens.
PHYSICAL CHARACTERISTICS:
- Color is colorless, white or off-white almost yellow.
- Luster is vitreous.
- Transparency: Crystals are transparent to translucent.
- Crystal System: Isometric; possibly of the gyroidal class 4 3 2.
- Crystal Habits include cubes, octahedrons and dodecahedrons. Complicated arborescent, snowflake-like and dendritic specimens are available. Crusts and coatings are more common.
- Cleavage is poor in one direction.
- Fracture is conchoidal to earthy.
- Hardness is 1.5 - 2
- Specific Gravity is 1.5 (very light).
- Streak is white.
- Associated Minerals include
sodium alum , sulfur and other fumarole minerals. - Notable Occurrences include Tadzhikistan; Mt. Vesuvius, Italy and Paricutin Volcano, Michoacan, Mexico.
- Best Field Indicators are crystal habit, associations, origin of formation, softness and density.