 MAGNESITE
MAGNESITE
- Chemistry: MgCO3, Magnesium Carbonate
- Class: Carbonate
- Group: Calcite
- Uses: an ore of magnesium
Specimens
PHYSICAL CHARACTERISTICS:
- Color is white or gray, also tinted yellow or brown.
- Luster is vitreous.
- Transparency crystals are translucent to transparent only in individual crystals.
- Crystal System is trigonal; bar 3 2/m
- Crystal Habits are usually massive forms such as lamellar, fiberous and course to fine grained rocks. Crystals are extremely rare, but when found are in the form of rhombohedrons or hexagonal prisms with a pinacoid termination.
- Cleavage is perfect in three directions forming rhombohedrons.
- Fracture is conchoidal to uneven.
- Hardness is 4 - 4.5.
- Specific Gravity is approximately 3.0 (average)
- Streak is white.
- Associated Minerals calcite, dolomite, aragonite, strontianite and serpentine.
- Other Characteristics: effervesces easily only in hot dilute hydrochloric acid.
- Notable Occurrences include Austria; Bahia, Brazil; Korea; China; California, USA and many European localities.
- Best Field Indicators are crystal habit, reaction to acid, occurrence and cleavage.
 Amethyst Galleries' Mineral Gallery MINERALS |

MAGNESITE specimen mgs-1
$ 20.00
$ 20.00
Dims: 7/8" x 5/8" x 5/8"
Wt: 4.9 g
Brumado Mine, Bahia, Brazil
This is one of the more unusual specimens that I have ever seen... It is purely composed of Magnesite, which is rarely found in crystal form. This specimen has crystal form, but it is unusual even for Magnesite! It is made up of 6 or 7 discernable crystals, all of which have an octahedral shape! The crsytals are transparent, and each has good form, with minimal damage on the visible faces and edges. The entire specimen is covered with small, uniform rust-colored pits. The whole thing is a mystery to me!

mgs-1 ($ 20.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-2
$ 75.00
$ 75.00
Dims: 2-3/4" x 2-1/2" x 2-1/4"
Wt: 7.08 oz
Mt. Brussilof Mine(near Radium), Brtish Columbia, Canada
This large, heavy specimen is packed with fan-shaped Magnesite blades! The largest measures about 1/2" wide, and like its siblings, has good clarity, clean faces with little damage(if any), and a sharp, curving edge. There may or may not be a matrix rock; it is covered with tiny crystals. There are lots of these crystals!


mgs-2 ($ 75.00)
Mt. Brussilof Mine(near Radium), Brtish Columbia, Canada

$ 125.00
Dims: 3-1/2" x 3-1/8" x 2-1/2"
Wt: 1 lb., 1.6 oz
Mt. Brussilof Mine(near Radium), Brtish Columbia, Canada
a visible matrix, but I cannot determine whether it is more Magnesite or calcite. This is a BIG specimen of a neat mineral!


Mt. Brussilof Mine(near Radium), Brtish Columbia, Canada

MAGNESITE specimen mgs-4
$ 45.00
$ 45.00
Dims: 1.9" x 1.8" x 1.7"(4.8 x 4.6 x 4.3 cm)
Wt: 2.55 oz.(72.4 g)
Mt. Brussilof Mine (near Radium), Brtish Columbia, Canada
Given that both the Magnesite and the associated dolomite on this specimen are colorless, dimly transparent, and have a pearly luster and similarly-shaped crystals, it is rather difficult (but not impossible) to discern one from the other. Their shapes are just different enough to tell them apart, and the dolomite crystals tend to be larger. There seem to be only three dolomite crystals on the specimen, one of which is heavily damaged. It grows off of the largest crystal, which measures 1.3 x 1.3 x 0.4"(3.3 x 3.3 x 1.0 cm). Their crystals are more tabular and don't have edges as sharp or as straight as those of the Magnesite. The Magnesiteite crystals also have a slightly higher luster. Other than these two minerals, there is no other material or host rock. This specimen warrants close examination.


mgs-4 ($ 45.00)
Mt. Brussilof Mine (near Radium), Brtish Columbia, Canada

MAGNESITE specimen mgs-5
$ 45.00
$ 45.00
Dims: 2.8" x 2.2" x 2.0" (7.1 x 5.6 x 5.1 cm)
Wt: 4.61 oz. (130.9 g)
Brumado Mine, Bahia, Brazil
This interesting specimen sheds some light on the confusion that I had concerning specimen MGS-1. It consists of a cluster of at least 15 rhombohedral Magenesite crystals, each of which is partly coated with a thin botryoidal crust of Hematite. The largest of these Magnesite crystals has dimensions of 0.7 x 0.7 x 0.6 (1.8 x 1.8 x 1.5 cm) and is noticeably damaged, as are 6 or 7 others. All have well-defined edges and where visible, smooth, clean faces that possess a pearly luster. They are colorless and dimly transparent, as they contain many internal fractures. The hematite crusts that coat them are made up of tiny nodules (not exceeding 2 mm in diameter) that have a red-brown color and a dull waxy luster. All of this rests on a base of broken crystalline or massive Magnesite that is stained a pale reddish color in some areas by rust.


mgs-5 ($ 45.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-7
$ 35.00
$ 35.00
Dims: 1.6 x 1.3 x 1.0" (4.1 x 3.3 x 2.5 cm)
Wt: 24.0 g
Brumado Mine, Bahia, Brazil
This thumbnail specimen consists of a cluster of heavily intergrown trigonal Magnesite rhombohedrons. These crystals have dimensions that do not exceed 1.0 x 0.5 x 0.4" (2.5 x 1.3 x 1.0 cm) and are in good condition, though several show noticeable damage and the largest is not quite complete. Where intact, however, they have excellent form, with sharp edges and clean faces that possess a bright vitreous luster. They are colorless, transparent, and moderately to dimly clear due to cloudy inclusions, intergrowth, and internal fracturing along their cleavage planes. Amidst the Magnesites stands a single hexagonal prismatic quartz crystal that is undamaged and shows excellent form. It is colorless, transparent, and has the standard vitreous luster. It does appear to have a slightly rusty coloration, but this is due to a thin film of rust that lies between the quartz crystal and the partly intergrown Magnesites. One of these extends an impressive distance into the quartz crystal, and is easily seen through its prism faces. There is no host rock present.

mgs-7 ($ 35.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-8
$ 36.00
$ 36.00
Dims: 3.2 x 2.0 x 0.8" (8.1 x 5.1 x 2.0 cm)
Wt: 2.33 oz. (66.3 g)
Brumado Mine, Bahia, Brazil
This small hand specimen consists of a crust made up of intergrown Magnesite crystals. These crystals do not exceed 0.4 x 0.4 x 0.4" (1.0 x 1.0 x 1.0 cm) in size and are generally in very good condition, though a few of them show some obvious damage. All have good trigonal rhombohedral form, with slightly-rounded but generally well-defined edges and smooth faces that possess a vitreous luster. The Magnesites are colorless, transparent, and moderately clear due to their intergrowth and the presence of cloudy inclusions and internal fractures. They appear to have a pale rust coloration, though, due to the fact that they are covered with a thin layer of hematite. Besides the Magnesite, there is a single hexagonal prismatic quartz crystal and a few tiny rhombohedral siderites scattered about the crust. The quartz crystal is rather difficult to see offhand due to its similiar color and luster to the Magnesite, but is readily visible with close examination (see the close-up image). The siderites, though tiny, are more readily visible due to their pale yellow-brown color. There is no host rock present on the specimen.


mgs-8 ($ 36.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-9
$ 28.00
$ 28.00
Dims: 1.6 x 1.0 x 0.9" (4.1 x 2.5 x 2.3 cm)
Wt: 18.7 g
Brumado Mine, Bahia, Brazil
This thumbanil specimen consists entirely of a cluster of trigonal rhombohedral Magnesite crystals. The largest of these crystals has dimensions of 0.7 x 0.6 x 0.5" (1.8 x 1.5 x 1.3 cm), and is not quite complete but shows no human-induced damage. Only one crystal in the cluster is damaged, and this damage basically consists of a small surface where a corner was cleaved off, and an adjacent internal fracture. All the crystals show exceptional form, with sharp edges and very clean faces that possess a bright vitreous luster. They are colorless, transparent, and moderately clear, as all contain internal fractures and flaws that run along their cleavage planes. Their faces are dusted with a very sparse layer of fine hematite, most of which is trapped just under the surface. There is no host rock present, and the cluster's base is made up of a jumble of more smaller, broken, and incomplete Magnesites.

mgs-9 ($ 28.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-10
$ 35.00
$ 35.00
Dims: 1.4 x 1.2 x 1.0" (3.6 x 3.1 x 2.5 cm)
Wt: 26.0 g
Brumado Mine, Bahia, Brazil
This small thumbnail specimen consists of a single rhombohedral Magnesite crystal that is mostly covered with a druse of scalenohedral calcites. The Magnesite has dimensions of 1.4 x 0.8 x 0.7" (3.6 x 2.0 x 1.8 cm) and is in good condition, showing only one small spot of human-induced damage besides the cleavage plane on one end. The other exposed faces, however, are natural and though they show some striations, they are clean and smooth, and possess a bright vitreous luster. The Magnesite is generally colorless, though at least one portion of it is visibly rust-stained. It is transparent and moderately clear, containing many cleavage-oriented flaws and internal fractures. The surrounding calcites are also in good condition, though many of them are partly cleaved. The largest of these is much larger than the others, with dimensions of 0.6 x 0.5 x 0.4" (1.5 x 1.3 x 1.0 cm) and an almost tabular form (see the close-up image). Most of the calcites have a pale rust-brown coloration due to included hematite, but all are transparent and quite clear. There is no host rock present.


mgs-10 ($ 35.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-11
$ 50.00
$ 50.00
Dims: 3.0 x 2.1 x 2.0" (7.6 x 5.3 x 5.1 cm)
Wt: 5.9 oz. (166 g)
Brumado Mine, Bahia, Brazil
This small hand specimen consists of a cluster of partly intergrown Magnesite rhombohedrons. The specimen is in moderately good condition, as most of the crystals show at least some minor damage, and many of the smaller ones at the base of the specimen are badly broken and incomplete. The largest of these has dimensions of 1.5 x 1.5 x 0.8" (3.8 x 3.8 x 2.0 cm), and like the others, has good rhombohedral form, with well-defined edges and flat, clean faces that possess a pearly luster. All are colorless, transparent, and dimly clear due to intense internal fracturing and some cloudy inclusions. They are also covered with crusts that are made up of scores of tiny, round and intergrown hematite nodules. These nodules generally have a red-brown coloration and a dull luster, but a few show a dark silver-gray coloration and dull metallic luster. There is also a large, incomplete crystal of a gray metallic mineral- it is in the center of the Magnesite cluster, and the crystals seem to have grown off of it. It must be made of hematite, as there is a lot of it elsewhere on the specimen, and it is too hard to be galena.


mgs-11 ($ 50.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-12
$ 36.00
$ 36.00
Dims: 2.4 x 1.4 x 0.8" (6.1 x 3.6 x 2.0 cm)
Wt: 1.78 oz. (50.6 g)
Brumado Mine, Bahia, Brazil
A cluster of intergrown Magnesite rhombohedra comprise this hand specimen. These crystals reach dimensions of 0.4 x 0.3 x 0.3" (1.1 x 0.8 x 0.7 cm) and are in good condition, though a few areas show obvious damage. Their trigonal form is very good, and all are transparent, moderately clear and possess a pearly luster. Though they are essentially colorless, a thin film of iron oxide gives them a pale rust-red tinge. Scattered about the cluster are several tiny hematite nodules that have a deep red-brown color and a dull, waxy luster.
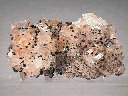
mgs-12 ($ 36.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-13
$ 90.00
$ 90.00
Dims: 2.9 x 2.5 x 1.5" (7.4 x 6.4 x 3.7 cm)
Wt: 6.1 oz. (174 g)
Brumado Mine, Bahia, Brazil
A single large Magnesite crystal makes up most of this specimen. The crystal is in very good condition, showing almost no fresh damage, and has dimensions of 2.6 x 2.5 x 1.5" (6.6 x 6.4 x 3.7 cm). Its rhombohedral form is also very good, and appears to contain evidence of twinning. It has the standard pearly luster and a milky-white color with a strong pink tinge, and is translucent and cloudy. Clusters of deep red garnets are intergrown with the crystal- I do not know exactly what their specie is- but there is no actual base or host rock present.


mgs-13 ($ 90.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-14
$ 30.00
$ 30.00
Dims: 2.1 x 1.4 x 0.7" (5.4 x 3.6 x 1.8 cm)
Wt: 0.6 oz. (16 g)
Brumado Mine, Bahia, Brazil
Dozens of tiny Magnesite crystals rest on the quartz base of this hand specimen. These crystals are in excellent condition - only a few are noticeably damaged - and none exceed 0.2" (4 mm) along any of their axes. All of them show good trigonal rhombohedral form, and all have a faint pink color due to hematite inclusions and the standard pearly luster. They are likewise transparent and moderately to very clear, containing hematite inclusions and internal cleavage planes. The quartz base is quite interesting; it is made up of several crystals that intersect with each other in such a way as to nearly confine themselves to one plane. They are transparent but are heavily included with a dull green substance that could be chlorite.

mgs-14 ($ 30.00)
Brumado Mine, Bahia, Brazil

MAGNESITE specimen mgs-15
$ 75.00
$ 75.00
dims mm=106.92x81.09x45.91
wt g=428
Mt. Brussilof Mine, near Radium, British Columbia, Canada
This hand specimen is essentially all magnesite, as an intergrown cluster of well-formed crystals. They are transparent, colorless, and have an unusual silky luster. Under a loupe, the surface is slightly irridescent in many colors.


mgs-15 ($ 75.00)
Mt. Brussilof Mine, near Radium, British Columbia, Canada